The Role of FDG PET/CT to Evaluation of Axillary Lymph Nodes after Neoadjuvant Chemotherapy in Breast Cancer
By Eda Tanrikulu Simsek1, Ezgi Coban1, Elif Atag1, Serkan Gungor2, Murat Sari1, Gunay Gurleyik3Affiliations
doi: 10.29271/jcpsp.2021.07.792ABSTRACT
Objective: To determine the diagnostic value of breast and axillary maximum standard uptake (SUVmax) values for predicting ypT0 and ypN0 separately.
Study Design: A descriptive study.
Place and Duration of Study: Department of Medical Oncology, Haydarpasa Numune Training and Research Hospital, between May 2017 and September 2020.
Methodology: Consecutive patients with operated breast cancer (BC) after neoadjuvant chemotherapy (NAC) were evaluated. SUVmax on FDG-PET/CT after NAC at both primary tumour (postSUVmax-T) and axillary lymph nodes (postSUVmax-N) were assessed to predict the ypT0 and the ypN0, respectively.
Results: Clinically meaningful correlation was detected between postSUVmax-N with ypN0 in patients with human epidermal receptor-positive (Her2+) and triple-negative (TN) BC (in Her2+ BC: r=0.596, p <0.001, in TN BC: r=0.782, p = 0.001). The postSUVmax-N predicted ypN0 with 90.5% positive predictive value (PPV) and 85.7% negative predictive value (NPV) in patients with Her2+ and TN BC. The postSUVmax-T predicted ypT0 with 87.5% PPV and 100% NPV in patients with TN BC (AUC: 0.938, P <0.01)
Conclusion: According to this study's findings, the FDG-PET/CT may be an alternative to sentinel lymph node biopsy (SNB) to protect patients from axillary lymph node dissection when the expected FNR of the SNB is high in patients with Her+ and TN BC.
Key Words: Breast cancer, FDG PET/CT, Neoadjuvant therapy.
INTRODUCTION
Today, the use of neoadjuvant chemotherapy (NAC) in the treatment of operable breast cancer (BC) is increasing. It is the initial treatment approach in 17-40% of patients, depending on the locoregional extent and biological subtype of the disease.1,2 Apart from the advantages of NAC, such as offering an increased chance for both breast-conserving surgery (BCS) and sentinel lymph node biopsy (SNB) for axillary staging, it is an in vivo susceptibility test for BC cells. It also allows for additional treatment planning for patients with residual tumours after NAC.3,4 The presence of pathological complete response (pCR) is considered a surrogate marker for survival outcomes.5
In recent studies with dual human epithelial receptor-2 (Her2) blockade in Her2-positive (Her2+) tumours and carboplatin-containing regimens in triple-negative (TN) tumours, pCR rates reached 68% and 80%, respectively.6,7
The complication rates of SNB for axillary staging are lower than axillary lymph node dissection (ALND). Therefore, SNB is the preferred method for axillary staging in early BC. Today, the majority of initially clinical node-positive (cN+) operable BC patients receive NAC, and a pCR on axillary lymph nodes (ALNs) can be achieved in 20% to 61% of these, with the degree of success varying according to specific molecular subtypes.8 Thus, SNB has become an option for initially cN+ patients who will undergo ALND, if they didn’t receive NAC. However, 63-100% sentinel node detection rates (DR) and 0-39 % false negativity rates (FNR) have been reported for SNB after NAC.9-11 Therefore, there is no established consensus for the staging of axilla especially in patients with initially cN+ with clinically node-negative after NAC (ycN-).9
Previously, several studies have examined the role of 18-fluorodeoxyglucose positron emission tomography/computerized tomography (FDG-PET/CT) in evaluating patients who received NAC for BC.12-16 The maximum standardised uptake value (SUVmax) at FDG-PET/CT after completion of NAC (postSUVmax) was previously shown to correlate with pCR.17 In this study, we evaluated whether postSUVmax could predict the absence of invasive cancer after completion of NAC in both breast (ypT0) and ALNs (ypN0) separately.
Thus, the aim of this study was to evaluate whether it was possible to omit surgical axillary staging by predicting pathological response with the help of FDG-PET/CT in BC; and to evaluate the role of FDG-PET/CT for predicting ypT0.
METHODOLOGY
All consecutive patients who were planned surgery after NAC for stage I-III BC in Hospital between May 2017 and September 2020 were searched. Data from 101 patients with BC were reviewed retrospectively. Patients who were evaluated with FDG-PET/CT after 14-to-21-days of the completion of NAC were included in this trial. Exclusion criteria were being younger than 18 years, presence of metastatic disease after NAC, not operated after NAC for any reason, and not being evaluated with FDG-PET/CT after NAC. The postSUVmax, which were measured at primary tumours and lymph nodes on performed FDG-PET/CT after completion of NAC, were defined as postSUVmax-T and postSUVmax-N, respectively. No FDG uptake in the primary tumour and ALNs on FDG-PET/CT was defined as complete response on FDG-PET/CT (FDG-PET/CT-CR). Fourteen patients were excluded from the study because they were not evaluated with FDG-PET/CT after NAC, and 2 patients were found to have metastases. All data were recorded from individual patient's health records (handwritten plus electronic files).
The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov/Shapiro-Wilk’s tests) to determine whether or not they were normally distributed. Qualitative data were given as frequencies and percentages, while quantitative as median, minimum and maximum. While investigating associations between non-normally distributed and/or ordinal variables, the correlation coefficients and their significance were calculated using the Spearman test. Receiver operating characteristic (ROC) curves of the postSUVmax to predict ypT0 and ypN0, were generated to determine the cut-off value that would yield the optimal sensitivity and specificity. The sensitivity was defined as the percentage of response on FDG-PET/CT with all the pathological responses. The specificity was defined as the percentage of non-response on FDG-PET/CT with all the pathological non-response. All statistical tests were 2-sided with a significance level of p<0.05. The data were analysed using the Statistical Package for the Social Sciences, Statistics V.22 (IBM Corp, Armonk, NY).
The 7th edition of the American Joint Committee on Cancer system was used for staging.18 All 85 tumors were immunohistochemically assessed for the presence of estrogen receptor (ER), progesterone receptor (PR), Her2, Ki-67 and histologic grade. The scoring recommendation from the American Society of Clinical Oncology/College of American Pathologists was used to evaluate ER, PR and Her2 status.19 The ypT0, ypN0 and pCR were defined as the absence of residual invasive carcinoma in the breast tissue, ALNs, both primary breast and lymph nodes, respectively.
RESULTS
A total of 85 patients with BC were evaluated (age: 27-69 years, median: 46). The patients were separated into three clinical subtypes as follows: ER-positive (ER+, at least 10% positivity for ER staining and Her2 negative) in 36 (42.4%) patients; Her2+ (ER positive or negative, and Her2 positive) in 34 (40%) patients and TN in 15 (17.6%) patients. The distribution of the overall stage was as follows: stage I, II, and III in 2 (2.3%), 43 (50.6%), and 40(47.1%) respectively. The patients were initially cN+; 84.7% in all patients (N0: 13, N1: 44, N2: 24; N3:4) and 85.7% in patients with Her2+ and TN BC (N0: 7, N1: 22, N2: 16; N3:4). All patients had received anthracycline and taxane-based NAC regimens. All 34 patients with Her2+ BC received trastuzumab. Among them, 15 patients received four cycles of pertuzumab. Fifty patients (58.8%) were premenopausal. SNB and ALND were performed on 33 and 52 patients, respectively. Among patients who were performed ALND, SNB was also performed on 14 patients. In patients with SNB; 1, 2, and at least 3 sentinel lymph nodes were removed in 4, 7, and 36 patients, respectively.
The NAC resulted in a pCR in 32 (37.6%) of all the patients. Positive correlation was detected between FDG-PET/CT-CR and pCR (p<0.001, r=0.551). The authors evaluated separately for each clinical subtype and, clinically meaningful correlation was detected between FDG-PET/CT-CR and pCR in patients with TN and Her+ BC (ER+: p=0.017, r=0.395; Her2+: p= 0.006, r=0.461; TN: p=0.001, r=0.764). Positive correlation was detected between postSUVmax-N, postSUVmax-T and ypN0, ypT0, respectively (p<0.001, r=0.504; p<0.001, r=0.569, respectively). When the authors evaluated separately for each clinical subtype, clinically meaningful correlation between postSUVmax-N with ypN0 (ER+: p=0.023, r=0.378, Her2+: p <0.001, r=0.596, TN: p = 0.001, r=0.782) and postSUVmax-T with ypT0 was detected in patients with TN and Her+ BC (ER+: p=0.032, r=0.359, Her2+: p = 0.001, r=0.543, TN: p <0.001, r=0.823). Patients with Her+ and TN BC were also evaluated together.
Among patients with initially cN+, 39(54.2%) of them were detected ypN0 after NAC. In patients with Her2+ and TN BC, in 32 of 42 (76.1%) patients who were initially cN+ were detected ypN0 after NAC.
PostSUVmax-T and postSUVmax-N were used as potential predictors of both ypT0 and ypN0 after NAC, respectively. Figure 1 shows the receiver operating characteristic (ROC) curves for ER+ and the combined group of Her2+ and TN. ROC curves of the postSUVmax-T to predict ypT0 revealed a threshold of postSUVmax-T was 0.5(AUC=0.784, %95CI: 0.685-0.883, p<0.001; Figure 1). ROC curves of the postSUVmax-N to predict ypN0 revealed a threshold of postSUVmax-N was 0.5 (AUC=0.702, %95CI: 0.582-0.822, p= 0.002; Figure 1). Since the only SUVmax value below 0.5 is ‘0’, we defined postSUVmax-T <0.5 as a complete response for postSUVmax-T (postSUVmax-T-CR) and postSUVmax-N <0.5 as a complete response for postSUVmax-N (postSUVmax-N-CR). Rates of post-SUVmax-T-CR, post-SUVmax-N-CR, ypT0 and ypN0 by clinical subtypes are summarised in Table I.
PostSUVmax-T and postSUVmax-N diagnostic value was evaluated to predict ypT0 and ypN0 by ROC curves, respectively, (Figure 1, Table II). Detected AUC's, sensitivities, specificities, positive predictive values (PPV), and negative predictive values (NPV) are summarised in Table II.
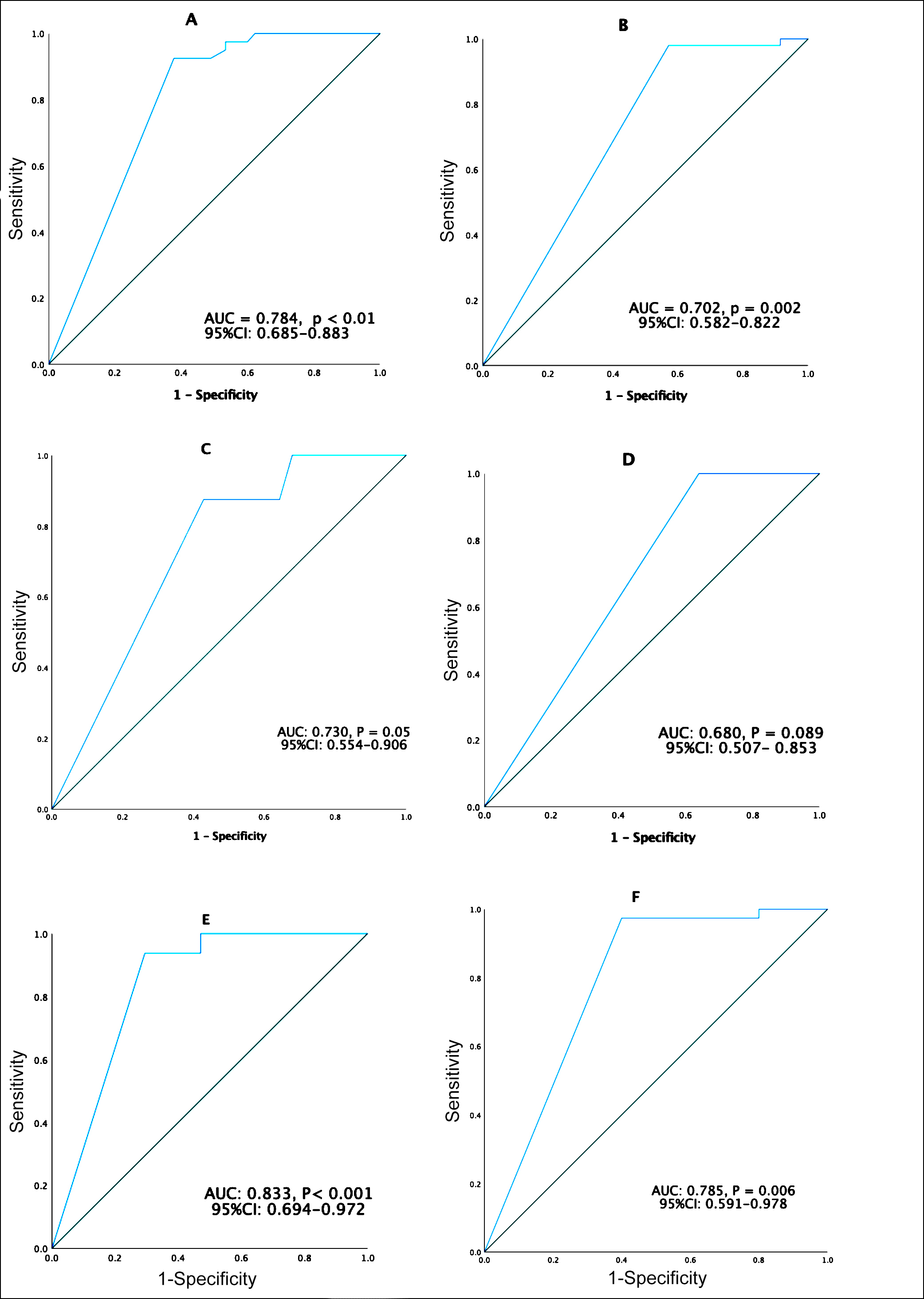 Figure 1: Receiver operating characteristics (ROC) curves of maximum standardized upatake value after neoajuvant chemotherapy on primary tumor (postSUVmax-T) and axillary lymph nodes (postSUVmax-N) for ypTO and ypNO: (A) PostSUVmax-T for ypTO in all patients (n=85). (B) PostSUVmax-N for ypNO in all patients (n=85). (C) PostSUVmax-T for ypTO in patients with estrogen receptionr-positive (ER+). Breast cancer (BC) (n=36). (D) PostSUVmax-N for ypNO in patients with ER+BC (n=36). (D) PostSUVmax-N for ypNO in patients with ER+BC (n=36). (E) PostSUVmax-T for ypTO in patients with human epidermal receptor-positive (Her2+) and triple negative (TN) BC (n=49). (F) PostSUVmax-N for ypNO in patients with Her-2+ and TN BC (n=49).
Figure 1: Receiver operating characteristics (ROC) curves of maximum standardized upatake value after neoajuvant chemotherapy on primary tumor (postSUVmax-T) and axillary lymph nodes (postSUVmax-N) for ypTO and ypNO: (A) PostSUVmax-T for ypTO in all patients (n=85). (B) PostSUVmax-N for ypNO in all patients (n=85). (C) PostSUVmax-T for ypTO in patients with estrogen receptionr-positive (ER+). Breast cancer (BC) (n=36). (D) PostSUVmax-N for ypNO in patients with ER+BC (n=36). (D) PostSUVmax-N for ypNO in patients with ER+BC (n=36). (E) PostSUVmax-T for ypTO in patients with human epidermal receptor-positive (Her2+) and triple negative (TN) BC (n=49). (F) PostSUVmax-N for ypNO in patients with Her-2+ and TN BC (n=49).
AUC: Area under the curve, CI: Confidence interval.
DISCUSSION
While NAC was used only to make BCS possible in the past, today it has gone beyond that and offers many advantages. One of its most important advantages is enabling SNB for axillary staging in the case of ycN0 after NAC in initially cN+. Thus, patients can be protected from the complications of ALND which may cause consequences that impair quality of life.20
Altered lymphatic drainage due to fibrotic changes after NAC and non-uniform regression in the tumour can make it difficult to detect sentinel lymph nodes. Therefore, axillary staging with SNB after NAC is controversial. Since the FNR of the SNB is about 6% in patients with initially clinically node-negative (cN-) and ycN- after NAC, most of the centres perform SNB for axillary staging after NAC in these patients.21Among initially cN+ patients, 20-61% convert to ypN0 depending on tumour type by NAC, with the highest responses in TN and Her2+ diseases.9 However, in patients with initially cN+, and ycN- after NAC, DR and FNR are nearly 80% and 15%, respectively.9 Therefore there is no established consensus regarding the staging of the axilla in these patients. This situation forced the researchers to develop alternative methods.9 However, these methods increase the costs, the need for trained and experienced personnel. Additionally, SNB is an effective procedure, if performed by experienced surgeons.9 It is not always possible to reach a surgeon with this competence in many centres. Although the complication rates are quite low; it should be discussed whether SNB is the ideal axillary staging method after NAC for the reasons stated above. In this study, it was found that in patients with Her2+ and TN BC, FDG-PET/CT predicts ypN0 with 90.5% PPV and 85.7% NPV, if there is no FDG uptake in ALNs after NAC. These are acceptable values. FDG-PET/CT may be an option to predict ypN0 in patients with TN and Her2+ BC.
In a previous study, it was shown that postSUVmax was closely correlated with pCR in patients with Her2+and TN BC.17 In contrast to Akimoto et al., this study evaluated the predictive value of postSUVmax-T and postSUVmax-N for ypT0 and ypN0 separately, respectively. Literature showed that after NAC was completed, the relationship between FDG-PET/CT and ypN0 was evaluated in only a few studies.22-24Among these studies, only the study of Vincente was prospective.24 In this study, the number of patients who had the node-positive disease was not specified. As a result of this trial, FDG-PET/CT after NAC has been found to be associated with lymph node response and prognosis.24 PPV, NPV of FDG-PET/CT to detect a residual tumour in the ALNs after NAC was 87% and 23%, respectively. In the present study, the high rate of cN+ patients at the beginning (84.7%) may have affected the results. It is believed that this study can be better adapted to clinical practice since the initial nodal status is specified in detail. The retrospective study by You et al. involved 99 patients with initial cN+ patients evaluated by FDG-PET/CT after NAC.23 PPV and NPV with FDG-PET/CT to predict residual tumours in the ALNs were found at 80% and 28%, respectively.
Table I: Rates of postSUVmax-T-CR, postSUVmax-N-CR, ypT0, and ypN0 by clinical subtypes.|
|
Total |
postSUVmax-T-CR |
ypT0 |
postSUVmax-N-CR |
ypN0 |
|
n |
n (%) |
n (%) |
n (%) |
n (%) |
|
|
ER+, |
36 |
19(52.8) |
8(22.2) |
27(75) |
11(30.6) |
|
Her2+ |
34 |
28(82.4) |
25(73.5) |
29(85.3) |
27(79.4) |
|
TN |
15 |
8(53.3) |
7(46.7) |
13(86.7) |
12(80) |
|
Combin |
49 |
36(73.5 |
32(65.3) |
42(85.7) |
39(79.6) |
|
Total, n (%) |
85 |
55(64.7) |
40(47.1) |
69(81.2) |
50(58.8) |
|
PET/CT: Positron emission tomography/computerised tomography, postSUVmax-T-CR: Complete response at primary tumour on PET/CT after neoadjuvant chemotherapy, postSUVmax-N-CR: Complete response at axillary lymph nodes on PET/CT after neoadjuvant chemotherapy, ER+: Estrogen receptor-positive, Her2 negative; Her2+: Human epidermal growth factor receptor-positive; TN: Triple-negative. |
|||||
Table II: Diagnostic performance of postSUVmax-T-CR to predict ypT0 and, postSUVmax-N-CR to predict ypN0.
|
Patient Group |
n |
Variable |
AUC |
%95 CI |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
|
All |
85 |
ypT0 |
0.784* |
0.685-0.883 |
92.5 |
60 |
67.3 |
90 |
|
|
|
ypN0 |
0.702* |
0.582-0.822 |
98 |
42.9 |
71 |
93.8 |
|
ER+ |
36 |
ypT0 |
0.730^ |
0.554-0.906 |
87.5 |
57.1 |
36.8 |
94.1 |
|
|
|
ypN0 |
0.680^ |
0.507-0.853 |
100 |
36 |
40.7 |
100 |
|
Her2+ |
34 |
ypT0 |
0.751** |
0.534-0.968 |
92 |
44.4 |
82.1 |
66.7 |
|
|
|
ypN0 |
0.762** |
0.525-0.999 |
96.3 |
57.1 |
89.7 |
80 |
|
TN |
15 |
ypT0 |
0.938* |
0.795-1 |
100 |
87.5 |
87.5 |
100 |
|
|
|
ypN0 |
0.833^ |
0.495-1 |
100 |
66.7 |
92.3 |
100 |
|
Combined Her2+ and TN |
49 |
ypT0 |
0.833* |
0.694-0.972 |
93.8 |
64.7 |
83.3 |
84.6 |
|
|
|
ypN0 |
0.785* |
0.591-0.978 |
97.4 |
60 |
90.5 |
85.7 |
|
PET/CT: Positron emission tomography/computed tomography; postSUVmax-T-CR: complete response at primary tumour on PET/CT after neoadjuvant chemotherapy; postSUVmax-N-CR: Complete response at axillary lymph nodes on PET/CT after neoadjuvant chemotherapy; ER+: Estrogen receptor-positive, Her2-negative; Her2+: human epidermal growth factor receptor-positive; TN: triple-negative; FNR: false negativity rate; *P <0.01; **P <0.05; ^p ≥ 0.0.5. |
||||||||
In that study, 28% of the patients who were initially cN+ became ypN0. In the present study, ypN0 was obtained in 54.2% (39/72) of the patients who were initially cN+. Moreover, this ratio was 76.2% (32/42) in patients with Her+ and TN BC. This situation may have caused the results of this study to differ. In the study by You et al., no molecular subgroup analysis was performed, and nodal stage distribution was not specified. Although patients who underwent SNB and/or ALND after NAC were evaluated, the rate and techniques in SNB were not specified. In this study, 61% of the patients were performed ALND and the remaining were performed SNB. Suspicious lymph node biopsy and clip placement were performed before NAC in all patients with initial cN+ disease, in case SNB was planned. It has been shown in previous studies these techniques significantly reduce FNR.25 Moreover, at least 2 sentinel lymph nodes were removed in 30 of 33 patients who underwent SNB. In the Z1071 study, performing axillary ultrasound after NAC and removing at least 2 sentinel lymph nodes has been shown to reduce FNR significantly.10 Therefore, the expected FNR is low due to the techniques used in SNB in our centre. In another study, determining residual tumour on ALNs with PET/CT after NAC in patients with initially cN1, PPV and NPV were detected 85.7% and 61.1%, respectively. However, only 38 patients were evaluated with FDG-PET/CT in this study.22
In this study, despite the PPV and NPV were 92.3% and %100 for ypN0, respectively, in patients with TN BC, there were statistically meaningless ROC curves. This is probably related to the low patient number in the TN group. On the other hand, pCR and ypN0 rates were high in both Her2+ and TN BC. Also, in these groups, clinically significant positive correlations were detected between pCR, ypN0, and FDG-PET/CT-CR, postSUVmax-N, respectively. Therefore, we also analysed by combining Her2 + and TN groups.
It was found that postSUVmax-T predicts ypT0 in patients with TN. Therefore, non-surgical follow-up in FDG-PET/CT-CR cases after NAC may be a recommendation in selected patients, such as the elderly, or patients with comorbid diseases in TN BC. On the other hand, prospective studies planned to evaluate the non-surgical follow-up by predicting pCR after NAC with various methods are ongoing.2 According to the results of this study, new studies can be planned according to pCR or ypN0 estimated by FDG-PET/CT in Her2+ and TN BC.
The small number of patients and its retrospective nature are the main limitations of this study. The fact that not all patients were evaluated with ALND is a limitation to this study. But the authors think that this is a limited effect on this study, as FNR is expected to be low due to the techniques used in this centre for SNB.
CONCLUSION
There is no established consensus on whether to perform SNB for axillary staging after NAC. In this study, it is detected that FDG-PET/CT predicts ypN0 with 90.5% PPV and 85.7% NPV in the Her2+ and TN BC. In the absence of adequate technical equipment and/or an experienced surgeon/radiologist, the FNR of SNB will inevitably increase. Based on the results of this study, in these cases, FDG-PET/CT may be used instead of SNB to predict ypN0 after NAC in Her2+ and TN BC patients. These findings should be evaluated with more comprehensive prospective studies to confirm the present results.
ETHICAL APPROVAL:
This study was approved by the local Ethics Committee (approval No. 2020/248).
PATIENTS’ CONSENT:
Because this study was retrospective, the patients’ consents were waived.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
ETS: Data collection, statistical analysis, data curation, writing original draft, writing reviews and editing.
EC, EA: Data collection, data curation, writing reviews.
SG: Data collection.
MS: Statistical analysis, data collection.
GG: Investigation, validation, supervision.
All the authors critically reviewed the final version of the manuscript and approved it for publication.
REFERENCES
- Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: Triple-negative and HER2+ subtypes. Ann Surg Oncol 2018; 25(8):2241-8. doi: 10.1245/ s10434-018-6531-5.
- Heil J, Kuerer HM, Pfob A, Rauch G, Sinn HP, Golatta M, et al. Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: Current evidence and future challenges. Ann Oncol 2020; 31(1):61-71. doi: 10.1016/ j.annonc.2019.10.012.
- Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017; 376(22):2147-59. doi: 10.1056/NEJMoa1612645.
- von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive her2-positive breast cancer. N Engl J Med 2019; 380(7):617-28. doi: 10.1056/NEJMoa1814017.
- Spring LM, Fell G, Arfe A, Sharma C, Greenup R, Reynolds KL, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin Cancer Res 2020; 26(12):2838-48. doi: 10.1158/1078-0432. CCR-19-3492.
- Wang C, Chen J, Xu X, Hu X, Kong D, Liang G, et al. Dual HER2 blockade in neoadjuvant treatment of HER2+ breast cancer: A meta-analysis and review. Technol Cancer Res Treat 2020; 19:1533033820960721. doi: 10.1177/15330 33820960721.
- Li ZY, Zhang Z, Cao XZ, Feng Y, Ren SS. Platinum-based neoadjuvant chemotherapy for triple-negative breast cancer: A systematic review and meta-analysis. J Int Med Res 2020; 48(10):300060520964340. doi: 10.1177/ 0300060520964340.
- Damin AP, Zancan M, Melo MP, Biazus JV. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with node-positive breast cancer: guiding a more selective axillary approach. Breast Cancer Res Treat 2021; 186(2):527-34. doi: 10.1007/s10549-020-06011-8.
- Ersoy YE, Kadioglu H. Review of novel sentinel lymph node biopsy techniques in breast cancer patients treated with neoadjuvant chemotherapy. Clin Breast Cancer 2018; 18(4):e555-e9. doi: 10.1016/j.clbc.2018.01.004.
- Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013; 310(14):1455-61. doi: 10.1001/jama. 2013.278932.
- Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol 2013; 14(7):609-18. doi: 10.1016/S1470- 2045(13)70166-9.
- Tian F, Shen G, Deng Y, Diao W, Jia Z. The accuracy of 18F-FDG-PET/CT in predicting the pathological response to neoadjuvant chemotherapy in patients with breast cancer: A meta-analysis and systematic review. Eur Radiol 2017; 27(11):4786-96. doi: 10.1007/s00330-017-4831-y.
- Mghanga FP, Lan X, Bakari KH, Li C, Zhang Y. Fluorine-18 fluorodeoxyglucose positron emission tomography-computed tomography in monitoring the response of breast cancer to neoadjuvant chemotherapy: A meta-analysis. Clin Breast Cancer 2013; 13(4):271-9. doi: 10.1016/j.clbc. 2013.02.003.
- Christin OL, Kuten J, Even-Sapir E, Klausner J, Menes TS. Node positive breast cancer: Concordance between baseline FDG-PET/CT and sentinel node assessment after neoadjuvant therapy. Surg Oncol 2019; 30:1-5. doi: 10.1016/j.suronc.2019.05.006.
- Koolen BB, Valdés Olmos RA, Wesseling J, Vogel WV, Vincent AD, Gilhuijs KG, et al. Early assessment of axillary response with ¹⁸F-FDG-PET/CT during neoadjuvant chemotherapy in stage II-III breast cancer: Implications for surgical management of the axilla. Ann Surg Oncol 2013; 20(7):2227-2235. doi: 10.1245/s10434-013-2902-0.
- Koolen BB, Donker M, Straver ME, van der Noordaa MEM, Rutgers EJT, Valdés Olmos RA, et al. Combined PET-CT and axillary lymph node marking with radioactive iodine seeds (MARI procedure) for tailored axillary treatment in node-positive breast cancer after neoadjuvant therapy. Br J Surg 2017; 104(9):1188-96. doi: 10.1002/bjs.10555.
- Akimoto E, Kadoya T, Kajitani K, Emi A, Shigematsu H, Ohara M, et al. Role of 18F-FDG-PET/CT in predicting prognosis of patients with breast cancer after neoadjuvant chemotherapy. Clin Breast Cancer 2018; 18(1):45-52. doi.org/10.1016/j.clbc.2017.09.006
- Edge SB, Compton CC. The american joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17(6): 1471-14. doi: 10.1245/s10434-010-0985-4.
- Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010; 28(16):2784-95. doi: 10.1200/JCO. 2009.25.6529.
- Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American college of surgeons oncology group trial Z0011. J Clin Oncol 2007; 25(24):3657-63. doi: 10.1200/JCO. 2006.07.4062.
- Geng C, Chen X, Pan X, Li J. The Feasibility and accuracy of sentinel lymph node biopsy in initially clinically node-negative breast cancer after neoadjuvant chemo-therapy: A systematic review and meta-analysis. PLoS One 2016; 11(9):e0162605. doi: 10.1371/journal. pone.0162605.
- Hieken TJ, Boughey JC, Jones KN, Shah SS, Glazebrook KN. Imaging response and residual metastatic axillary lymph node disease after neoadjuvant chemotherapy for primary breast cancer. Ann Surg Oncol 2013; 20(10):3199-3204. doi: 10.1245/s10434-013-3118-z.
- You S, Kang DK, Jung YS, An YS, Jeon GS, Kim TH. Evaluation of lymph node status after neoadjuvant chemotherapy in breast cancer patients: Comparison of diagnostic performance of ultrasound, MRI and ¹⁸F-FDG-PET/CT. Br J Radiol 2015; 88(1052):20150143. doi: 10.1259/bjr.20150143.
- García Vicente AM, Amo-Salas M, Relea Calatayud F, Muñoz Sánchez M, Pena Pardo FJ, Jiménez Londoño GA, et al. Prognostic role of early and end-of-neoadjuvant treatment 18F-FDG-PET/CT in patients with breast cancer. Clin Nucl Med 2016; 41(7):e313-22. doi: 10.1097/RLU.0000000 000001191.
- Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: Implementation of targeted axillary dissection. J Clin Oncol 2016; 34(10):1072-8. doi: 10.1200/JCO.2015. 64.0094.