The Predictive Value of First-trimester Thiol/Disulfide Homeostasis in Preeclampsia
By Habibe Ayvaci Tasan1, Gultekin Adanas Aydin2, Erbil Cakar1, Nazan Tarhan Usal1, Ebru Cogendez1, Enis Ozkaya1, Cemile Bicer3, Ozcan Erel3Affiliations
doi: 10.29271/jcpsp.2021.04.405ABSTRACT
Objective: To investigate the efficacy of first-trimester thiol/disulfide homeostasis (t/dh), a new oxidative stress marker, in predicting preeclampsia.
Study Design: Prospective cohort study.
Place and Duration of Study: Department of Obstetrics and Gynecology, University of Health Sciences, Zeynep Kamil Women and Children Diseases Training and Research Hospital, Istanbul, Turkey, Department of Obstetrics and Gynecology, Bursa Yüksek Ihtisas Training and Research Hospital, Bursa, Turkey, from March 2016 to February 2019.
Methodology: In this multi-centre,serum samples of women with839 singleton pregnancies were collected between 11+0 to 13+6gestational weeks. A total of 215 singleton pregnant women were included in the study. The patient group consisted of 38 women, who were diagnosed with preeclampsia; while the control group consisted of 177 healthy pregnant women without any complication during pregnancy and after delivery. Totalthiol (TT) was estimated by the sum of existing thiol groups and reduced thiol groups (S-S and -SH). After the native thiols (-SH) and (TT) were determined, the disulfide (-SS) amounts, disulfide/total thiol percent ratios (-SS/-SH + -SS), disulfide/native thiol percent ratios (-SS/-SH), and native thiol/total thiol percent ratios (-SH/-SH + -SS) were calculated.
Results: There were no statistically significant differences between the groups in terms of[(-SH), (TT), (-SS), (-SS/-SH), (-SS/-SH + -SS), and (-SH/-SH + -SS)] six t/dh variables(p>0.05).The first-trimester body mass index (BMI) was statistically different between the two groups (p<0.001). In the receiver operating characteristic curve analysis, none of the concentrations of thiol levels and ratios was found to have a significant predictive value for preeclampsia. The BMI was a significant predictor for preeclampsia (area under curve: 0.749, p<0.001).
Conclusion: Maternal serum t/dh at 11+0 to 13+6 weeks of gestation does not predict preeclampsia and t/dh may be the consequence rather than a cause in the pathogenesis of preeclampsia.
Key Words: First-trimester, Preeclampsia, Sulfhydrylcompounds, Thiols.
INTRODUCTION
Oxidative stress is the excessive amount of reactive oxygen species (ROS) released into the circulation. Excessive ROS products induce vascular damage in the placenta, leading to impaired trophoblast differentiation.1
Therefore, secondary to the increased oxidative stress, adverse perinatal outcomes, particularly preeclampsia (PE), preterm delivery, fetal growth restriction (FGR), stillbirth, intrauterine fetal demise, and small for gestational age (SGA) can be more frequently seen.
Thiolsplay a critical role in oxidative stress. The target area of ROS is thiol groups in aminoacids and proteins. Plasma thiol pool is mainly composed of albumin and protein thiols and a less amount of low-molecular-weight thiols such as cysteinyl-glycine, cysteine (Cys), homocysteine, glutathione, and γ- glutamyl-cysteine.2 When the oxidative stress increases, thiol groups in proteins and in low-molecular-weight thiols (Cys residues) react with each other to form reversible disulfides. These disulfide bonds can be reduced to thiol groups and, therefore, t/dh can be maintained.3 Until 2014, low-molecular-weight thiols and disulfides Cys, cystine (CySS), and glutathione and glutathione disulfide (GSG and GSSG) were measured. By 2014, however, a new,fully-automated method developed by Erel and Neselioglu,4 has been utilised to measure thiol and oxide thiol reserve for dynamic t/dh in the body.
Earlier detection of high-risk patients for adverse perinatal outcomes is of utmost importance to reduce maternal and fetal morbidity using preventive therapeutic measures. Currently, several serum biomarkers are in use to predict adverse perinatal outcomes,particularly PE prior to clinical onset of the disease.5,6
In previous clinical studies, increased oxidative stress and decreased antioxidant system have been blamed for complicated pregnancies such as PE, FGRand miscarriage.7-9 However, there is no study available in the literature investigating the predictive value of t/dh in PE. The present study, therefore, aimed to examine the efficacy of first-trimester t/dh, a new oxidative stress marker, in predicting PE.
METHODOLOGY
This multi-centre, prospective, cohort study was conducted at the Department of Obstetrics and Gynecology, University of Health Sciences, Zeynep Kamil Women and Children Diseases Training and Research Hospital, Istanbul, Turkey and Department of Obstetrics and Gynecology,Bursa Yüksek İhtisas Training and Research Hospital, Bursa, Turkey, between March 2016 and February 2019. A written informed consent was obtained from each participant prior to study entry. The study protocol was approved by the local Ethics Committee of Hospital (Date: 18/11/2015, No. 21-03).
In total, serum samples of women with839 singleton pregnancies were collected in the first- trimester between 11+0 to 13+6 weeks of gestation during the first-trimester aneuploidy screening. After birth, obstetric outcomes were recorded from hospital database systems and clinical records where they gave birth. A total of 215 singleton pregnant women were included in the study. The patient group consisted of 38 women who were diagnosed with PE, while the control group consisted of 177 healthy pregnant women without any complication during pregnancy and after delivery.Of the PE patients, 30 were diagnosed with late-onset PE. The t/dh was also compared between the late-onset PE patients and the control group.
Pregnant women using acetylsalicylic acid or anticoagulants or in whom these agents were prescribed at the first examination were excluded from the study. In addition, those having Type I or Type II diabetes mellitus, connective tissue disorders, chronic renal and/or liver disease, hyper/hypothyroidism, hematological diseases, multiple pregnancies, or smoking habit were also excluded from the control group.
Demographic data including maternal age, BMI (weight in kilograms / height in meters2), gravidity, paritywere recorded for each patient at the time of sample collection. The gestational age was calculated by the first day of the last menstrual period and confirmed by the first-trimester crown rump lengthmeasurementusing the obstetric ultrasonography.
Preeclampsia was diagnosed, when the systolic/diastolic blood pressure was ≥140/90 mmHg and presence of proteinuria ≥300 mg in 24-h urine specimen.10 Late-onset PE was defined as diagnosis of PE after 34 weeks of gestation.
T/dh levels were assessed from venous blood samples which obtained as 3mL for the first-trimester aneuploidy screening. Initial step for the analysis wassample centrifugationat 3,500 rpm for 10 mins to separate the serum, following this process all serum samples were kept at -80° until analysis. Transfer of this samples to the laboratory was carried out by Styrofoam boxes containing dry ice to prevent thawing and maintain temperature. For the measurement of serum disulfide/thiol homeostasis, the novel, fully-automated, spectrophotometricmethod described by Erel and Neselioglu,4 was used via the Cobasc501 instrument (Roche Diagnostics, Mannheim, Germany). Values of serum disulfide homeostasis were shown with the unit of µmol per liter.Reduction of disulfide bond to form the free functional thiol groups was obtained by sodium borohydride (NaBH4). Following this process the reductants of NaBH4 that were not used completely removed by formaldehyde to prevent further reduction of 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB) was prevented. The sum of the thiol groups present and the reduced thiol groups (SS and -SH) gave the total thiol (TT). The dynamic amount of disulfide was automatically calculated to be half the difference between the (TT) and natural thiols (-) values. SH). After estimating (-SH) and (TT), disulfide (-SS) amounts, disulfide / total thiol percent ratios (-SS / -SH + -SS), disulfide / natural thiol percent ratios (-SS / -SH) and percent ratios of natural thiol / total thiol (-SH / -SH + -SS) were calculated.
The sample size was calculated with 95% power with an alfa (α) error of 0.05 as 98 [1/4 allocation: study (n=20), control (n=78)] based on a previous study of Akkaya et al.11 The incidence of PE was accepted as 4 to 5% and it was planned to collect 840 blood samples.
Statistical analysis was performed using the SPSS for Windows version 17.0 software (SPSS Inc., Chicago, IL, USA). Descriptive data were expressed in mean ± standard deviation (SD), median (Q1-Q3) or number andpercentage. The Chi-square test was used to compare categorical variables between the groups. An independent samplest-test was used for parametric data,while the Mann-Whitney U-test was used for non-parametric data. The receiver operating characteristic (ROC) curve was performed to estimate the predictive value of each parameter of first-trimester t/dh and BMI for PE. A p value of <0.05 was considered statistically significant.
RESULTS
Of the total of 215 cases, the median age was 29.7 (26-32) years in the PE group and 24.2 (25-35.3) years in the control group, without statistically significantly different. However, the median BMI values were significantly lower in the control group (p<0.001).
Table I: Demographic characteristics of the study population.|
Variable |
Control group n=177 median (Q1-Q3) |
PE group n=38 median (Q1-Q3) |
p-value |
|
Age (year) |
29 (26-32) |
31.5 (25-35.3) |
0.156 |
|
BMI (kg/m2) |
24.2 (21.8-27.1) |
28.9 (25.9-34.3) |
<0.001 |
|
Gravidity |
2 (1-3) |
2 (1-4) |
0.022 |
|
Parity |
1 (0-1) |
1 (0-2) |
0.013 |
|
Living fetus |
1 (0-1) |
1 (0-2) |
0.003 |
|
Gestational age at the time of serum sample collection (day) |
88 (83-92) |
87 (84.8-91) |
0.791 |
|
Birth week (week) |
39 (38-40) |
36 (35-38) |
<0.001 |
|
Birth weight (gram) |
3350 (3090-3600) |
2815 (1975-3122.5) |
<0.001 |
|
Data are given in median (Q1-Q3) values, unless otherwise stated. Bold values indicate statistical significance at p<0.05. PE, preeclampsia; BMI, body mass index. |
|||
Table II: Serum thiol/disulfide levels control and PE group.
|
Variable |
Control group n=177 mean ± SD or median (Q1-Q3) |
PE group n=38 mean ± SD or median (Q1-Q3) |
p-value |
|
Native thiol (-SH) (µmol/L) |
387.7 ± 49.7 |
377.6 ± 44.8 |
0.248a |
|
Total thiol (µmol/L) |
436.1 ± 49.5 |
424.1 ± 40.0 |
0.165a |
|
Disulfide (-SS) (µmol/L) |
24.1 ± 9.5 |
22.5 ±7.4 |
0.344a |
|
Disulfide/native thiol (-SS/-SH) |
6.1 (4.7-7.8) |
6.0 (4.5-7.1) |
0.533b |
|
Disulfide/total thiol (-SS/-SH + -SS) |
5.4 (4.3-6.7) |
5.4 (4.1-6.2) |
0.499b |
|
Native thiol/total thiol (-SH/-SH + -SS) |
89.2 (86.5-91.5) |
89.8 (87.8-91.8) |
0.349b |
|
aIndependent samples t-test; bMann-Whitney U-test. Data are given in mean ± SD or median (Q1-Q3) values, unless otherwise stated. PE, preeclampsia; SD, standard deviation. |
|||
Table III: Serum thiol/disulfide levels in control and late-onset PE group.
|
Variable |
Control group n=177 mean ± SD or median (Q1-Q3) |
Late-onset PE group n=30 mean ± SD or median (Q1-Q3) |
p-value |
|
Native thiol (-SH) (µmol/L) |
387.7 ± 49.7 |
372.4 ± 44.1 |
0.115a |
|
Total thiol (µmol/L) |
436.1 ± 49.5 |
420.0 ± 40.1 |
0.094a |
|
Disulfide (-SS) (µmol/L) |
24.1 ± 9.5 |
22.9 ± 7.4 |
0.505a |
|
Disulfide/native thiol (-SS/-SH) |
6.1 (4.7-7.8) |
5.5 (4.5-7.1) |
0.658b |
|
Disulfide/total thiol |
5.4 (4.3-6.7) |
5.0 (4.2-6.2) |
0.621b |
|
Native thiol/total thiol (-SH/-SH + -SS) |
89.2 (86.5-91.5) |
89.8 (87.6-91.7) |
0.732b |
|
aIndependent samples t-test; bMann-Whitney U-test. Data are given in mean ± SD or median (Q1-Q3) values, unless otherwise stated. PE, preeclampsia; SD, standard deviation. |
|||
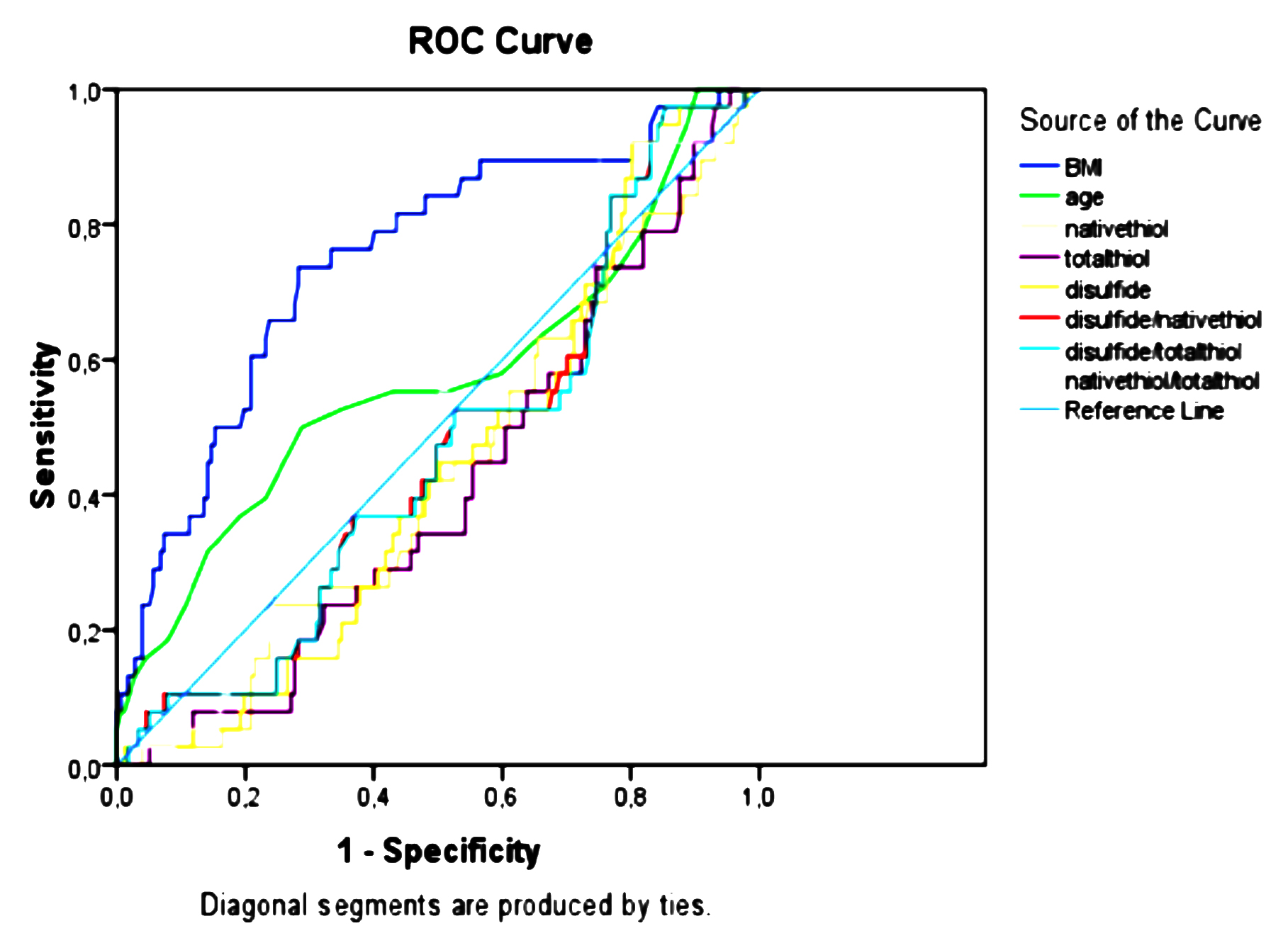 Figure 1: The receiver operating characteristic curve analysis.
Figure 1: The receiver operating characteristic curve analysis.
Gestational week at birth and fetal birth weight were statistically significantly higher in favour of the control group (p<0.001). There was a significant difference in the number of gravidity and parity and living fetus between the groups (Table I).
Serum values of t/dh of blood samples obtained from both groups between the 11+0 and 13+6 weeks of gestation are shown in Table II. Accordingly, there were no statistically significant differences between the groups in terms of the six t/dh parameter values (p>0.05). Furthermore, no statistically significant difference was found in the serum values of t/dh of the first trimester between the late-onset PEpatients and control groups (p>0.05, Table III).
In the ROC analysis, none of the concentrations of thiol levels and ratios was found to have a significant predictive value for preeclampsia (Figure 1). Only, the BMI was found to be a significant predictor for preeclampsia ([AUC]:0.749, p<0.001, 95% C.I: 0.66-0.84).
DISCUSSION
Early detection of high-risk groups for PE theoretically provides an opportunity for close follow-up or planning prophylactic medical interventions. Based on the current evidence, the first-trimester screening tests using serum markers or ultrasonography are not sufficient to provide positive predictive value for detecting adverse pregnancy outcomes. A better understanding of pathophysiology in early pregnancy would provide an insight into this.
In recent years, a number of proteins and anti-angiogenetic factors such as PAPP-A, free beta-human chorionic hormone (β-hCG), PIGF, and sFlt-1 have been used as placental biomarkers. Thesebiomarkers have also a prediction rate of 5% and false-positivity rate of 44 to 92% in detecting early-onset PE.12
In their study, Baschat et al. evaluated PE risk in 2,441 women using the first-trimester screening and concluded that high BMI and low PAPP-A multiple of the median (MoM) values were correlated with high PE rates.13 In the present study, similarly, the first-trimester BMI values were found to be significantly higher in the PE group. Although this subject is out of the scope of this study, it may support that the sample size of this study and patient selection are suitable to achieve accurate statistical interferences.
The sFlt-1, a protein produced from the hypoxic placenta,is also used as a predictor for PE. The sFlt-1 levels have been shown to increase dramatically in patientswith PE; however, plasma levels of its ligands (vascular endothelial growth factor and PlGF) tend to decrease.14
In a study, Odibo et al. investigated metabolomic biomarkers in the first-trimester PE screening and reported an increased concentration of seven organic molecules (two hydroxyl carnitine esters and five amino acids) in the maternal blood of PE cases in the second half of pregnancy, compared to controls.15 In this study, t/dh was also evaluated as a marker of maternal oxidative stressduring the 11+0 to 13+6 weeks of pregnancy. Accordingly, there were no statistically significant differences for each of the t/dh parameter values between the PE patients and healthy controls.
Anderson et al. reported that HbF and A1M could be used as new oxidative stress biomarkers for predicting early and late-onset PE with 5% false positivity and 69% prediction rate.12 In the present study, systemic oxidative stress was investigated as in the study by Anderson et al.16 however, here t/dh was used as the oxidative stress marker. This study results showed no statistically significant differences for each of the six t/dh parameter values between the PE patients and healthy controls.
Preeclampsia may be early- or late-onset. In early-onset PE, the main cause is defective placentation process. In late-onset PE, maternal genetic predisposition and metabolic disorders have been mostly blamed.In the clinical setting, maternal serum parameters yield different results for both subtypes.17 In a study evaluating total oxidant status (TOS) and total antioxidant status (TAS) in placental tissue specimens of early- and late-onset PE and healthy controls, the mean TOS was found to be significantly higher, while the mean TAS was significantly lower in the PE cases, specifically in the early-onset PE group.18
In this study, we observed no statistically significant differences for each of the six t/dh parameter values between the late-onset PE patients and healthy controls. The fact that previous first-trimester prediction models were more sensitive in early-onset PE than late-onset PE,19 and that previous studies using oxidative stress biomarkers showed more significant results in early-onset PE than late-onset PE can be the main reason for the lack of a significant difference in the t/dh between the study groups. Kyozuka et al. performed metabolome analysis on first-trimester maternal serum samples. Although they reported that three metabolites could be potential biomarkers for early onset hypertensive diseases, the metabolic profiles of healthy pregnant women and pregnant women with early onset hypertensive disease were found similar.20 However, the predictive value of first-trimester t/dh in early-onset PE may be another research topic in further studies.
To the best of authors’ knowledge, this is the first study to examine serum t/dh as an oxidative stress marker in the first trimester in predicting PE. In a prospective study, Akkaya et al. measured dynamic thiol/disulfide concentrations in women with suspected missed abortion in the first trimester of pregnancy and reported a significant difference in the t/dh variables in patients in whom missed abortion developed later.11
Currently, whether t/dh is a cause or a consequence in PE still remains controversial. Thiol groups are oxidized with ROS in the environment and form disulfide bonds. The conversion of thiols to disulfide is the initial step in ROS-mediated protein oxidation and any abnormality in oxidizing- anti-oxidizing balance can result in a number of complications during pregnancy such as PE. In their study, Genc et al. compared serum oxidative stress biomarkers and antioxidant parameters between 10th-14th and 20th-24th weeks of gestation in patients PE versus healthy controls and reported that oxidative damage already started before the onset of clinical symptoms.21 This study results showed that serum t/dh at the 11+0 to 13+6 weeks of gestation was similar between PE cases and healthy controls who gave birth without an adverse obstetric outcome. This can be attributed to the absence of maternal endothelial damage at the time that we collected blood samples. It is also well-known that compounds with thiol groups are antioxidants and have a critical role in opposing oxidative stress owing to their reductive properties.22 This can be the main reason for the discrepancy between the present study results and previous studies in terms of antioxidant biomarkers.
Nonetheless, there are some limitations to the present study. In this study, the authors were unable to collect serum samples in each trimester, and to evaluate t/dh throughout the pregnancy. Therefore, further large-scale, well-designed studies evaluating t/dh in all trimesters are needed to establish a definite conclusion.
CONCLUSION
These preliminary results show that maternal serum t/dh at 11+0 to 13+6 weeks of gestation is not useful in predicting PE, suggesting that t/dh may be the consequence rather than a cause in the pathogenesis of PE. The emerging shift of t/dh after the diagnosis of PE should be further investigated in large-scale and well-designed studies to shed light into the role of t/dh in the pathophysiological changes of PE throughout the pregnancy.
ETHICAL APPROVAL:
The study was approved by the Institutional Ethics Committee of Bursa Yüksek İhtisas Training and Research Hospital, Bursa Turkey.
PATIENTS' CONSENT:
All study participants gave a signed written consent before inclusion into study.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
HAT: Design of the work, data collection, critical revision of the article.
GAA: Data collection.
EÇ: Data collection, critical revision of the article.
NTU: Design of the work, data collection, data analysis.
EC: Design of the work, drafting article.
EÖ: Data analysis.
CB, ÖE: Laboratory analysis.
All the authors read and approved the final manuscript.
REFERENCES
- Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med 1999; 222(3):222–35. doi: 10.1046/j.1525-1373.1999.d01-139.x.
- Turell L, Radi R, Alvarez B. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radical Biology and Medicine 2013; 65:244-53. Doi: 10.1016/j.freeradbiomed.2013.05.050.
- Jones DP, Liang Y. Measuring the poise of thiol/disulfide couples in vivo. Free Radical Biology and Medicine 2009; 47(10):1329-38.doi: 10.1016/j.freeradbiomed. 2009.08. 021.
- Erel O, Neselioglu S.A novel and automated assay for thiol/ disulphide homeostasis. Clin Biochem2014; 47(18): 326-32. doi: 10.1016/j.clinbiochem.2014.09.026.
- Saxena AR, Seely EW, Rich-Edwards JW, Wilkins-Haug LE, Karumanchi SA,McElrath TF. First trimester PAPP-A levels correlate with sFlt-1 levels longitudinally in pregnant women with and without preeclampsia. BMC Pregnancy and Childbirth 2013; 13:85.doi: 10.1186/1471-2393-13-85.
- Gupta S, Goyal M, Verma D,Sharma A, Bharadwaj N, Kabra M,et al. Adverse pregnancy outcome in patients with low pregnancy-associated plasma protein-A: The indian experience. J ObstetGynaecol Res 2015; 41(7):1003-8. doi: 10.1111/ jog.12662.
- Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update 2006; 12(6): 747-55.doi: 10.1093/humupd/dml016.
- Schneider D, Hernández C, Farías M,Uauy R, Krause BJ, Casanello P,et al. Oxidative stress as common trait of endothelial dysfunction in chorionic arteries from fetuses with IUGR and LGA. Placenta 2015; 36(5):552-8. doi: 10.1016/j.placenta.2015.02.003.
- Biberoglu E, Biberoglu K, Kirbas A, Daglar K, Genc M, Avci A, et al. Circulating and myometrial markers of oxidative stress in pregnant women with fetal growth restriction. J ObstetGynaecol Res 2016; 42(1):29-35. doi: 10.1111/jog. 12857.
- Gestational hypertension and preeclampsia: ACOG practice bulletin, number 222. Obstet Gynecol2020;135(6):e237-e260.
- Akkaya H, Uysal G, Büke B, Gök G, Erel Ö, Karakükçü Ç. Evaluation of thiol/disulphide homeostasis as a novel predictor testing tool of early pregnancy viability. Taiwan J Obstet Gynecol 2018; 57(3):427-43.doi: 10.1016/j.tjog. 2018. 04.034.
- Anderson UD, Gram M, Åkerström B. First trimester prediction of preeclampsia. CurrHypertens Rep 2015; 17(9):584.
- Baschat AA, Magder LS, Doyle LE,Atlas RO, Jenkins CB, Blitzer MG. Prediction of preeclampsia utilising the first trimester screening examination. Am J Obstet Gynecol 2014; 211(5): 514.e1-7.doi: 10.1016/j.ajog.2014.04.018.
- Ilekis JV, Tsilou E, Fisher S,Abrahams VM, Soares MJ, Cross JC, et al. Placental origins of adverse pregnancy outcomes: Potential molecular targets: Anexecutive workshop summary of the eunice kennedy shriver national institute of child health and human development. Am J Obstet Gynecol 2016; 215(1):S1-S46. doi: 10.1016/j.ajog. 2016.03.001.
- Odibo AO, Goetzinger KR, Odibo L, Cahill AG, Macones GA, Nelson DM, et al. First-trimester prediction of preeclampsia using metabolomic biomarkers: A discovery phase study. PrenatDiagn 2011; 31(10):990-4.doi: 10.1002/pd.2822.
- Anderson UD, Olsson MG, Rutardottir S,Centlow M, Kristensen KH, Isberg PE,et al. Fetal hemoglobin and alpha1-microglobulinas first- and early second-trimester predictive biomarkers for preeclampsia. Am J Obstet Gynecol 2011; 204(6): 520.e1-5.doi: 10.1016/j.ajog. 2011.01.058.
- Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: Pathophysiologyandclinicalimplications. BMJ 2019; 366:l2381. doi: 10.1136/bmj.l2381.
- Daglar K, Kirbas A, Timur H, OzturkInal Z, Danisman N. Placentallevels of total oxidativeand anti-oxidativestatus, ADAMTS-12 anddecorin in early- andlate-onset severe preeclampsia. J Matern Fetal Neonatal Med 2016; 29(24):4059‐64.doi: 10.3109/14767058.2016.1154942.
- Nevalainen J, Korpimaki T, Kouru H, Sairanen M, Ryynanen M. Performance of first trimesterbio chemical markers and meanarterial pressure in prediction of early-onset pre-eclampsia. Metabolism 2017; 75:6-15. doi: 10.1016/ j.metabol.2017.07.004.
- Kyozuka H, Fukuda T, Murata T, Endo Y, Kanno A, Yasuda S, et al. Comprehensive metabolomic analysis of first-trimester serum identifies biomarkers of early-onset hypertensive disorder of pregnancy. Sci Rep 2020; 10(1): 13857.doi: 10.1038/s41598-020-70974-3.
- Genc H, Uzun H, Benian A, Simsek G, Gelisgen R, Madazli R, et al. Evaluation of oxidativestressmarkers in firsttrimester forassessment of preeclampsia risk. Arch Gynecol Obstet 2011; 284(6):1367‐73.doi: 10.1007/ s00404-011-1865-2.
- Winther JR, Thorpe C. Quantification of thiols anddisulfides. BiochimBiophysActa 2014; 1840(2):838-46.doi: 10.1016/ j.bbagen.2013.03.031.