Surgical Treatment of Gastric GIST: Feasibility of Laparoscopic Resection and Postoperative Outcome
By Costache Mircea Florin, Filip Bogdan, Lupascu Cristian, Trofin Ana Maria, Danciu Mihai, Scripcariu ViorelAffiliations
doi: 10.29271/jcpsp.2020.05.519ABSTRACT
Objective: The aim of this study is to evaluate clinical manifestations and the possibility of laparoscopic treatment of large gastric stromal tumors.
Study Design: A case-series.
Place and Duration of the Study: Ist Surgical Unit, Saint Spiridon Hospital Iasi and the Ist Oncological Surgical Unit, Regional Institute of Oncology Iasi, Romania, from May 2012 to May 2017.
Methodology: Patients with gastrointestinal stromal tumors (GISTs) who underwent surgery were selected. The clinical manifestations, type of surgical treatment, postoperative assessment of the risk factors, and follow-up for at least two years were analysed. Tumors longer than 5 cm were classified as large tumors.
Results: Forty-eight patients with GIST were seen, of which 25 (52.08%) had gastric tumor. Nine laparoscopic resections and 16 open interventions were performed on tumors up to 10 cm in size. The histopathological examination revealed 10 patients (40%) as class risk 3a / 3b. Complications were more frequent in open surgery, with an overall rate of 43.75% (7 patients) vs. 33.33% (3 patients) in laparoscopic surgery.
Conclusions: Laparoscopic surgical procedures performed in experienced centres can also be applied to gastric tumors over 5 cm in well-selected cases without repercussions on survival.
Key Words: Laparoscopy, Gastrointestinal stromal tumor, Gastric, Resection.
INTRODUCTION
Developed from mesenchymal tissue, Cajal cells or their precursors, gastrointestinal stromal tumors (GISTs) are the most common non-epithelial tumor of the gastrointestinal tract1. The term GIST was first used by Mazur and Clark in 1983,2 although it was not recognised until 1990. CD34 was the first clinical marker used to distinguish GISTs from true GI leiomyoma, leiomyosarcoma or schwannama in clinical practice.3 GISTs are most common in the stomach (50-60%) and the small intestine (30-35%) and occur less frequently in colorectal tract (5%) and esophagus (<1%). GISTs in adults almost never disseminate through lymphatic vessels and metastases, have a predilection for the liver, while locoregional recurrences often occur in oment or peritoneum.
Most patients have vague and non-specific symptoms. Digestive hemorrhage, abdominal pain or abdominal discomfort, dyspepsia, dysphagia, nausea, vomiting even occlusion,4 constipation or diarrhea, and a palpable tumor are some of the most common symptoms.
There is essential to correlate the clinical aspect with image on CT scan.5 An over-expression of the highly sensitive and specific KIT marker (CD117) led to the exact diagnosis of GIST for the first time. Together CD117 and DOG1 diagnose nearly 100% of GIST.6 There is a correlation between CD34 positivity and tumour site and between CD117 and the morphology of the tumour.7
Due to concerns about tumor rupture, through the widespread use of laparoscopic surgery, ESMO recommendations for laparoscopic surgery are only for GISTs ≤2 cm. GISTs can be manipulated without directly taking the tumorous mass with the brush, and various techniques have been described in order to prevent tumor rupture such as hanging the nearby gastric wall for traction, the use of endoscopic clips, plastic bags, and other techniques.8 Classic open surgery is also indicated for small tumors (even 2 cm) when they are very well vascularised, fragile, or near the esophagus junction.
The objective of this study was to evaluate clinical manifestations and the possibility of laparoscopic treatment of large gastric stromal tumors.
METHODOLOGY
This prospective study was conducted between May 2012 and May 2017 at Regional Institute of Oncology, Iasi and First Clinic of Surgery St. Spiridon Hospital, Iasi, Romania. All patients diagnosed with GIST were considered, in which surgery was performed.
Table I. Surgical management for gastric GIST in the current series.|
Type of surgery |
Nr. (%) |
Mean time (min) |
Blood loss Intraoperative (ml) |
R0 |
R1/tumour rupture |
R2 |
|
Laparoscopic resection |
9 (36) |
75 |
50 |
8 |
1 |
0 |
|
Open surgery - wedge resection |
12 (48) |
93 |
85 |
12 |
0 |
0 |
|
Open distal gastrectomy |
3 (12) |
125 |
175 |
3 |
0 |
0 |
|
Open multiorgan resection |
1 (4) |
185 |
350 |
0 |
1 |
0 |
Table II: Comparison of postoperative complications in laparoscopic versus surgery resection.
|
Postoperative complications |
Number of patients |
Percent (%) |
|
|
Laparoscopic resection (9 cases) |
Haemorrhage |
1 |
11.11 |
|
Nausea, vomiting |
1 |
11.11 |
|
|
Acute urinary retention |
1 |
11.11 |
|
|
Open surgery (16 cases) |
Haemorrhage |
2 |
12.5 |
|
Nausea, vomiting |
3 |
18.75 |
|
|
Wound infection |
1 |
6.25 |
|
|
Cardiac disorders |
1 |
6.25 |
|
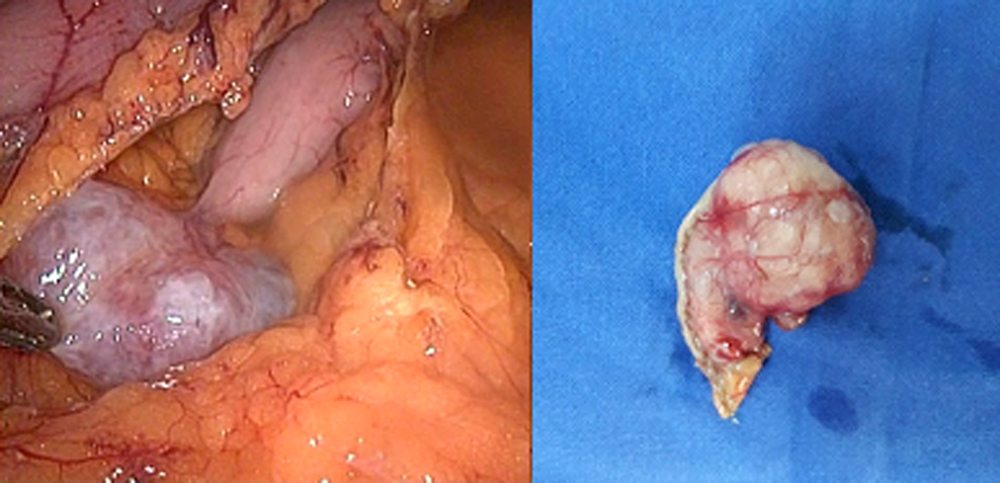 Figure1: Dissection of gastrocolic ligament and identification of the gastric GIST.
Figure1: Dissection of gastrocolic ligament and identification of the gastric GIST.
Finally only the patients with primary resected gastric GISTs over 18 years, without any other synchronous tumours, were selected. Those with metastasis, being first referred to the Oncology department and receiving the Imatinib treatment were excluded from this study. The purpose of surgical treatment was to completely resect the tumour. In all cases, patients’ consents were obtained. The risk classification was obtained using the Miettinen scale of the Armed Forces Institute of Pathology, which include the tumour size and the mitotic index.9
From a surgical point of view, patients were operated by open and laparoscopic procedures by several surgical teams with the same level of expertise. For the laparoscopic approach, the four-trocar French position was used. The first trocar of 10 mm for optics in the umbilical scar, the 5 mm trocar under xiphoid process, one of 10 mm in the right upper quadrant, and other one of 12 mm in the left upper quadrant. The intraabdominal pressure was 12 mm Hg. The tumor was visually inspected and identified with grasper forceps. In cases where the tumor could not be palpated, perioperative endoscopy was used. Immediate morbidity and mortality were studied up to 30 days. The data was collected on a database in Excel Microsoft Office 365. The study was approved from Ethics Board of University of Medicine and Pharmacy, Gr.T. Popa, Iasi.
RESULTS
A total number of 48 patients diagnosed with gastrointestinal stromal tumors (GIST), were identified. The mean age was 61.83 (±14.33) years ranging between 32 and 94 years. The gender ratio was 20 F : 28 M. Depending on the location of the tumor, the most common were gastric GISTs (n = 25, 52.08%), followed by small bowel GISTs (n = 13, 27.08%).
Of the 48 patients, extragastric GIST patients were eliminated, leaving 25 gastric GIST patients with an average age of 62.2 years, located in upper gastric region (n = 12, 48%), gastric body (n = 9, 36% ) and antrum (n = 4, 16%). The gender ratio was 15 F:10 M.
Regarding the clinical manifestations of patients, the most frequent clinical signs were abdominal pain in 9 cases (36%), upper digestive haemorrhage in 7 cases (28%), palpable abdominal mass 3 cases (12%); and in 6 patients (24%), there was no clinical sign. Most common comorbidities were hypertension (14 cases), diabetes (9 cases), chronic heart disease and pulmonary disease (8 cases each), chronic renal failure (3 cases); and one female patient was previously treated for a breast cancer. On admission, the mean haemoglobin level was 10.8 g/dl (7.2-14) and albumin level was 3 g/dl (2.3-3.9).
All 25 patients with gastric GIST were operated. The intervention and characteristics of intervention are mentioned in Table I. Because of the tumour size, laparoscopic resection was possible only in 9 cases (36%); and 3 of these were for tumors larger than 5 cm. Postoperative complications can be seen in Table II.
The histopathological and immunohistochemical characteristics of the gastric tumors in the study showed that most tumors were stage 3a (6 cases) and 3b (4 cases), followed by stage 5 (4 cases), 6a (4cases) and 6b (3 cases). The mean size of the tumour was 9.83. In this study, there were 2 recurrences in patients with R1 resection at 4 months postoperatively and at 14 months in a case of a R0 resection, but in an agressive tumour.
DISCUSSION
The most common locations of GISTs are at the gastric level (55.6%) (10) with a better prognosis than the other locations. In this study, the proportion of gastric GISTs was 52.08%, similar to the values reported by other studies.10,11 The proximal location (corpus and upper stomach) was the majority (84%), as previously reported (2/3 of the gastric tumor).12
As a clinical presentation, GISTs can embrace a variety of forms: from asymptomatic small tumors, abdominal discomfort up to digestive hemorrhages, or even palpable abdominal tumors. De Matteo reported an average of 6 months from the onset of the symptomatology to the diagnostic time.13 In this case series, gastric GISTs started most commonly with diffuse abdominal pain (36%), followed by digestive hemorrhage (28%) and asymptomatic patients (24%). Digestive hemorrhage is produced by necrosis and ulceration of the mucosa covering the tumor, resulting in bleeding from the broken vessels.
As others have noted,11 there is no ideal staging and prognosis system, but the proposed ones stratify GISTs to fit the tumor into a prognostic class and thus be able to follow a protocol for treatment. Here, the authors used the prognostic system proposed by the Armed Forces Institute of Pathology (AFIP), which seemed be the most accurate. Most cases in this study (40%) were in low or moderate class risk 3a / 3b. Although the mean tumor size in this study was 9.83 cm, the literature data showed a non-homogeneous distribution with mean dimensions from 1.5-2 cm to 18.5 cm; and only one study reported tumours with mean diameter of 8.78±5.6 cm.14 There were no patients with hepatic metastases, peritoneal or other localisations at the time of diagnosis; similar data was found in literature.15
The main objective of surgical treatment is R0 resection; and this can be done both laparoscopically (10-15 years ago, the tumor size limit being 2 cm, currently laparoscopic resection is being considered safe in tumors up to 5 cm)16 as well as open procedure. Otani suggested a 5 cm size as an indication for laparoscopic resection.17 Some series reported a superiority of laparoscopic resection for GIST versus open surgery even over 5 cm, by resuming rapid oral intake, lower blood loss and low inflammatory response.18 Long-term results with respect to recurrence and survival were comparable to open surgery. De Vogelaere et al. published a study with laparoscopic surgical treatment on a large series of gastric GISTs of 2-11 cm demonstrating that this type of approach is safe with low morbidity and no long-term relapse.19 The present study confirms this, the essential part being the way of manipulating the tumor and avoiding capsular rupture. Tumor rupture is considered an R1 resection just like confirmation of negative margins of resection less than 1 mm of tumor. It is considered that the laparoscopic manipulation of tumors between 2 and 5 cm increases the risk of tumor rupture. In this study, a 4.3 cm tumor approached laparoscopically ruptured during dissection, considering the resection R1, even if it had negative margins of resection. It is important to select patients for laparoscopic surgery by assessing the preoperative tumor size and the existence of a small pedicle even to GIST larger than 5 cm.
The rate of relapse is an important factor in the evolution of GISTs. The presence of residual tumor tissue is significantly correlated with early relapse and reduced survival. The negative effect of macroscopic residual tumor tissue is well known, demonstrating a significantly higher 5-year survival rate in patients with complete GIST resection (42% vs. 9%).20 In this study, there were 2 recurrences in patients with R1 resection at 4 months postoperatively and at 14 months in a case of a R0 resection, but in an agressive tumour. In fact, the average time of progression of the tumor even in patients treated with imatinib is approximately 2 years .
CONCLUSION
Although multiple studies comparing different surgical techniques do not recommend laparoscopic resection for large GISTs, this study suggests that gastric stromal intestinal tumors can be addressed safely through laparoscopic surgical technique even in large dimensions (up to 10 cm), provided they are approached in a multidisciplinary centre with experience in the treatment of these tumors.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
SV, LC: Contributed in study design.
CMF, TAM: Contributed in data aquisition, analysis and data interpretation.
CMF, FB: Drafted the paper.
DM, SV: Made final approval of the version.
REFERENCES
- Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): A review. Eur J Cancer 2002; 38(Suppl 5):S39-51.
- Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983; 7:507-19.
- Miettinen M, Virolainen M, Maarit Sarlomo R. Gastrointestinal stromal tumors-value of CD34 antigen in their identification and separation from true leiomyomas and schwannomas. Am J Surg Pathol 1995; 19:207-16.
- Humenansky KM, Gulati R. Small bowel gastrointestinal stromal tumor disguised as an adnexal mass: A source for midgut volvulus. J Surg Case Rep 2018; 2018:157.
- Danti G, Addeo G, Cozzi D, Maggialetti N, Lanzetta MM, Frezzetti G, et al. Relationship between diagnostic imaging features and prognostic outcomes in gastrointestinal stromal tumors (GIST). Acta Biomed 2019; 90:9-19.
- Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol 2009; 33:1401-8.
- Hashmi AA, Faraz M, Nauman Z, Qureshi MU, Hashmi SK, Waseem HF, et al. Clinicopathologic features and prognostic grouping of gastrointestinal stromal tumors (GISTs) in Pakistani patients: an institutional perspective. BMC Res Notes 2018; 11:457.
- Qiu G, Wang J, Che X, He S, Wei C, Li X, et al. Laparoscopic versus open resection of gastric gastrointestinal stromal tumors larger than 5 cm: A single-center, retrospective study. Surg Innov 2017; 24:582-9.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol 2006; 23:70-83.
- Soreide K, Sandvik OM, Soreide JA, Giljaca V, Jureckova A, Bulusu VR. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 2016; 40:39-46.
- Ud Din N, Ahmad Z, Arshad H, Idrees R, Kayani N. Gastrointestinal stromal tumors: A clinicopathologic and risk stratification study of 255 cases from pakistan and review of literature. Asian Pac J Cancer Prev 2015; 16:4873-80.
- Yang Z, Feng X, Zhang P, Chen T, Qiu H, Zhou Z, et al. Clinicopathological features and prognosis of 276 cases of primary small gastric gastrointestinal stromal tumours. Surg Endosc 2019; 33:2982-90.
- DeMatteo RP. The GIST of targeted cancer therapy: a tumor (gastrointestinal stromal tumor), A mutated gene (c-kit), and a molecular inhibitor (STI571). Ann Surg Oncol 2002; 9:831-9.
- Jumniensuk C, Charoenpitakchai M. Gastrointestinal stromal tumor: Clinicopathological characteristics and pathologic prognostic analysis. World J Surg Oncol 2018; 16:231.
- Alecu L, Tulin A, Enciu O, Barbulescu M, Ursut B, Obrocea F. Gastrointestinal stromal tumors - Diagnosis and surgical treatment. Chirurgia (Bucur) 2015; 110:525-9.
- Kim JJ, Lim JY, Nguyen SQ. Laparoscopic resection of gastrointestinal stromal tumors: Does laparoscopic surgery provide an adequate oncologic resection? World J Gastrointest Endosc 2017; 9:448-55.
- Otani Y, Furukawa T, Yoshida M, Saikawa Y, Wada N, Ueda M, et al. Operative indications for relatively small (2-5 cm) gastrointestinal stromal tumor of the stomach based on analysis of 60 operated cases. Surgery 2006; 139:484-92.
- Lian X, Feng F, Guo M, Cai L, Liu Z, Liu S, et al. Meta-analysis comparing laparoscopic versus open resection for gastric gastrointestinal stromal tumors larger than 5 cm. BMC Cancer 2017; 17:760.
- De Vogelaere K, Van Loo I, Peters O, Hoorens A, Haentjens P, Delvaux G. Laparoscopic resection of gastric gastrointestinal stromal tumors (GIST) is safe and effective, irrespective of tumor size. Surg Endosc 2012; 26:2339-45.
- Pierie JP, Choudry U, Muzikansky A, Yeap BY, Souba WW, Ott MJ. The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg 2001; 136:383-9.