Risk of Infertility Index in Women with Polycystic Ovarian Syndrome
By Munazzah Rafique1, Ayesha Nuzhat2, Dania Al-Jaroudi1Affiliations
doi: 10.29271/jcpsp.2020.11.1188ABSTRACT
Objective: To construct the risk of infertility index for women with polycystic ovarian syndrome, that can be used to assess the prognostic factors for conception; and categorise them in mild, moderate and high-risk groups.
Study Design: Descriptive study.
Place and Duration of Study: King Fahad Medical City, Riyadh, Saudi Arabia from March 2017 to February 2018.
Methodology: Fifty women who were diagnosed as polycystic ovarian syndrome were selected by simple randomisation. After initial scrutiny, 39 women with all clinical, biochemical and sonographic features of Rotterdam criteria were included. Patients with congenital adrenal hyperplasia (CAH) and premature ovarian insufficiency (POI) were excluded. Risk of infertility (RII) scale was used to assess the score in women with polycystic ovarian syndrome (PCOS) and classify them into different risk categories like mild (score ≤7), moderate (score 8-10) and severe (score >10).
Results: There were 5.1% cases with a mild score, 61.5% with a moderate score, and 33.3% with a severe score. For cases in the high-risk group, 89.8% of women had no pregnancy. RII with the cut-off point of 12.5 had a sensitivity of 100%, and specificity of 88.2%, for the defined outcome. Area under the curve was 0.553 and 95% CI was 48.3% (lower bound) and 62.3% (upper bound).
Conclusion: PCOS patients can be categorised in mild, moderate and high-risk depending on the score on RII scale. The patients with higher score of RII had fewer chances of pregnancy with assisted reproductive technologies (ART). Once the intensity of the risk of PCOS is identified, focused management can be implied thereby reducing the duration of infertility and cost of the treatment.
Key Words: Risk, Female infertility, Index, Polycystic ovarian syndrome, Assisted reproductive technology.
INTRODUCTION
Infertility is the prevalent chronic health problem characterised by the inability of a couple to conceive after one year . Eighty-five percent of couples conceive in first year of unprotected intercourse, if no clinical evaluation is indicated.1 Boivin et al. reported infertility to be 5-15% after analysing data from more than 25 countries from different parts of the world.2 In Haifa, KSA it is found that the prevalence of infertility was 18.93%.3 The commonest cause of medically treatable infertility is polycystic ovarian syndrome (PCOS).4 In a study conducted on Saudi girls aged 18–28 years, the estimated prevalence of PCOS was observed to be 53.7%, which is very high.5
Rotterdam ESHRE/ASRM consensus group diagnostic criteria have been recommended for the purpose of research and therapeutics. Two out of the three criteria are required to confirm PCOS, these include clinical features – oligomenorrhoea/amenorrhoea, hyperandrogenic signs and symptoms; biochemical – hyperandrogenic chemistry, and radiological – ultrasound findings of PCO, either ovarian volume: one or two ovaries >10 cm3 or one or two ovaries >12 follicle.6,7 In KSA, although studies have been conducted to establish the hormonal, metabolic and clinical profile for Saudi women with polycystic ovary syndrome; in our knowledge, no study has been conducted yet using the recently approved consensus on prognostic criteria of PCOS.8
The construction of a scoring system will optimise the ART treatment of the patients according to their categorisation in groups. By categorising the risk of infertility index (RII), patients can be classified into low, mild, and severe risk groups. Accordingly, patients will be prioritised according to the risk and appropriate management, which could be tailored for each patient thus; ensuring cost-effective treatment and lesser burden on healthcare resources. This study was intended to construct a scoring system for women with WHO type 2 anovulation (PCOS) that can be used to assess the prognostic factors for conception; and categorise them in mild, moderate, and high risk groups.
METHODOLOGY
After taking IRB approval (IRB No. 17-023) retrospectively, cases from 2017 to 2018 at Reproductive Endocrinology and Infertility Medicine Department of King Fahad Medical City, Riyadh, Saudi Arabia were included. Inclusion criteria was all women with age 19-40 years referred to the infertility centre having clinical, biochemical and ultrasound evidence of polycystic ovarian syndrome. Besides, women who fulfilled the inclusion criteria i.e PCOS WHO group 2 ovarian dysfunction as per Rotterdam criteria were included.6
Patients with incomplete records were excluded. Moreover, women having hyperandrogenism due to other causes like adrenal hyperplasia, adrenal tumor, ovarian hyperthecosis, ovarian tumour, thyroid problems and using any medications were also excluded from study. Data was obtained from electronic medical records of the patients. All the data were entered into a data collection sheet, which comprised of two sections: The first section detailed the demographic characteristics of the patients, while the second section collected clinical information like age, duration of infertility, BMI, menstrual disorder, hirsutism, acne, galactorrhea, any significant past history and previous infertility treatment. The physical examination findings were recorded as per Ferryman-Gallway score. A transvaginal ultrasound was done to assess the number of follicles and the volume in each ovary.
The laboratory investigations included: serum FSH, LH, TSH, T4, prolactin, testosterone, SHBG levels, androgen-free index (AFI), 17 OH-progesterone, estrogen E2, HBA1C, serum insulin, and vitamin D. Data including the expectant (including lifestyle modifications and exercise) treatment, ovulation induction and timely intercourse (OI-TI), intrauterine insemination (IUI) and in-vitro fertilisation (IVF) were also noted. The patients were followed up for expected outcome that were either pregnancy, no pregnancy, complication like ovarian hyperstimulation syndrome (OHSS) were recorded.
Records of the selected patients were coded and anonymised, followed by copying the data of the parameters in Excel sheet for SPSS analysis. For each patient, the score was calculated according to the proposed classification and finally categorising them into mild, moderate and severe risk groups.
Data was analysed using Statistical Package for Social Sciences (SPSS) software version 22 (Illinois, USA). Frequencies and percentages of demographic and clinical characteristics of the patients were taken as categorical variables. Table I describes the determinants of RII scoring and classification into different categories.
Index of infertility scale was calculated using above Table to assess the score in women with polycystic ovarian syndrome (WHO group 2 ovarian dysfunction) and classified them into different risk categories. of mild (score ≤7), moderate (score 8-10), and severe (score >10). The authors developed this scale after literature review of a study by Zarchi et al. who had originally used risk of malignancy scale for detection of pelvic malignancies compared with pathological reports.9
The face validity for the scale was determined by experts in the field at the study centre including the chairperson of infertility department after having systematic search of the literature. Content validity was approved by the chairperson of ethical board along with the literature review. Spearman’s correlation was carried out between the total score obtained after adding up items in the RII with the outcome (conception/pregnancy). Cronbach’s alpha was calculated to determine the internal consistency that was 0.4. The number of patients was used to determine Spearman’s correlation and not the percentage. ROC analysis was done in SPSS to determine the cutoff point for sensitivity and specificity.
RESULTS
There were 50 women who presented as PCOS, who were selected randomly to include in the study. After initial scrutiny, 39 women who had either all clinical, biochemical and sonographic criteria fulfilling two out of three Rotterdam criteria were included. Spearman’s correlation between total score obtained after adding up items in the RII with outcome (conception/pregnancy) was calculated and a weak correlation of .132 with p-value .424 (not significant) was observed. Table II depicts the clinical data of PCOS cases.
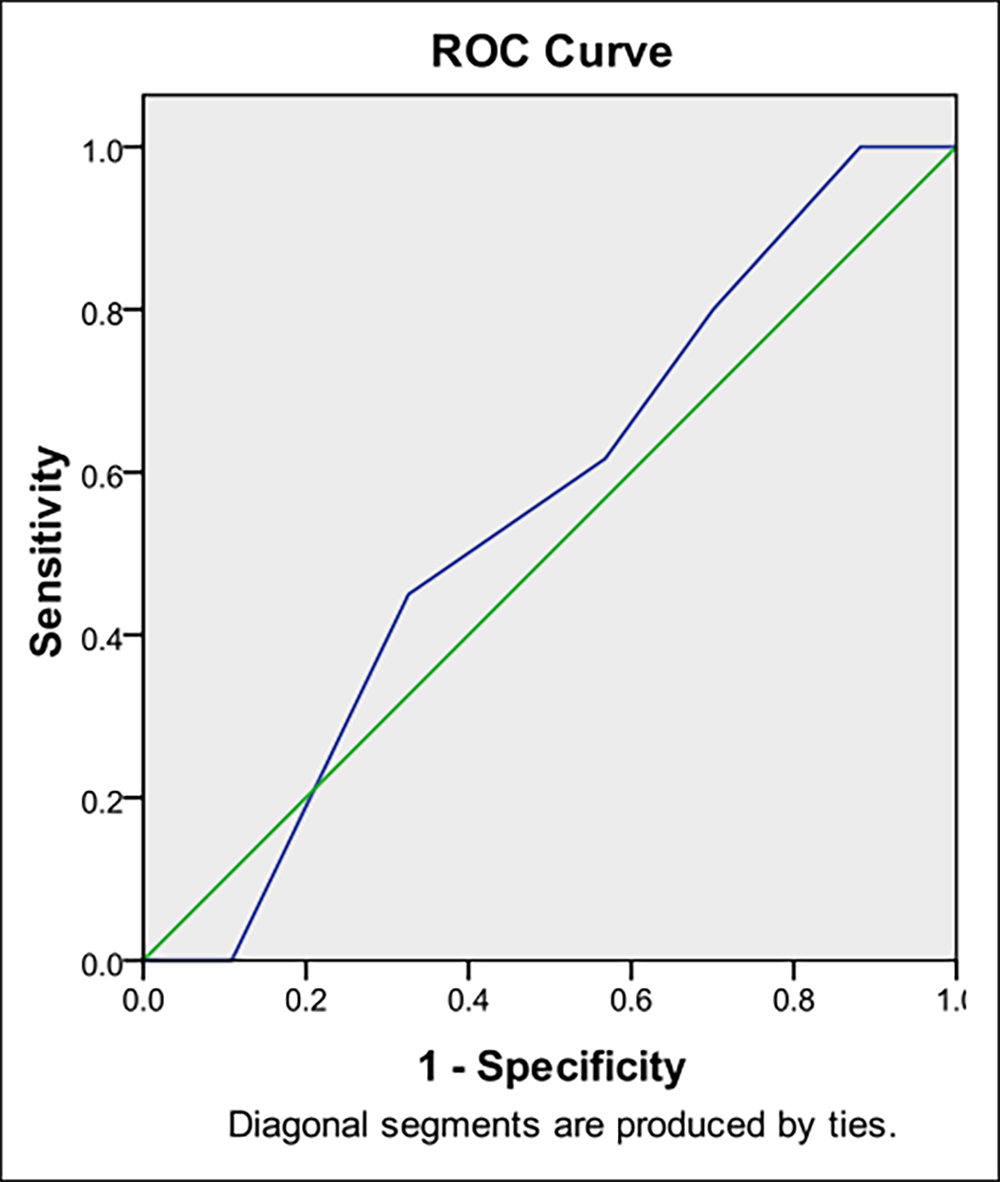 Figure 1: ROC curve shows the possible cutoff for RII.
Figure 1: ROC curve shows the possible cutoff for RII.
Table I: Scoring for risk of infertility index for PCO patients.
|
Score |
1 |
2 |
|
Age |
≤35 |
>35 |
|
BMI |
≤30 |
>30 |
|
Oligo/anovulation |
Oligomenorrhoea (35–182 days) |
Amenorrhea (>182 days) |
|
Hyperandrogenemia/Hirsutism |
(Ferriman-Gallwey score ≤8) |
Ferriman-Gallwey score >8) |
|
Biochemical |
Free Androgen Index ≤4.5 |
Free Androgen Index >4.5 |
|
Ultrasound findings: PCO on transvaginal sonography |
Volume: one or two ovaries ≤10 cm3 |
Volume: one or two ovaries >10 cm3 |
|
|
One or two ovaries ≤12 follicle |
One or two ovaries >12 follicle |
Table II: Clinical data of PCOS cases (n=39).
|
Characteristics |
Categories |
N=% |
|
Age (years) |
≤35 >35 |
30 (76.9%) 9 (23.1%) |
|
BMI (Kg/m2 ) |
≤30 >30 Missing |
18 (46.2%) 20 (51.3%) 1 (2.6%) |
|
Periods |
Oligomenorrhoea Amenorrhoea |
12 (30.8%) 27 (69.2%) |
|
Hirsutism |
Ferriman-Gallwey score ≤8 Ferriman–Gallwey score 8 |
27 (69.2%) 12 (30.8%) |
|
Free androgen index |
≤4.5 >4.5 Missing |
12 (30.8%) 14 (35.9) 13 (33.3%) |
|
Ovarian dimension |
≤ 10cm3 >10cm3 Missing |
13 (33.3%) 22 (56.4%) 4 (10.3%) |
|
Ovarian follicles |
≤12 >12 Missing |
5 (12.8%) 33 (84.6%) 1 (2.6%) |
|
Intervention |
Expectant Intrauterine Insemination (IUI) In-vitro fertilization (IVF) Ovulation Induction (OI) |
16 (41%) 2 (5.1%) 13 (33.3%) 8 (20.5%) |
|
Outcome |
No pregnancy Pregnancy Complication (OHSS) Missing |
32 (82.1%) 4 (10.3%) 2 (5.1%) 1 (2.6%) |
Figure 1 ROC curve shows the graphical way of connection between clinical sensitivity and specificity for every possible cutoff for RII index. The area under ROC curve gave an idea about benefit of using index and overall performance of RII. A value greater than or equal to this cutoff was treated as positive, while less than cutoff value as unfavourable. RII with different cutoff point is shown in the figure with minium sensitivity of 45.0% and specificity of 32.6% at cutoff of 9.5 and maximum sensitivity of 100%, and specificity of 88.2% at cutoff of 12.5 for outcome of PCOS. The area under the curve was 0.553 and 95% CI was 48.3% (lower bound) and 62.3% (upper bound). The cutoff value giving the highest TPR + TNR (sensitivity + specificity) is 12.5 in the study. So any value of RII more than 12.5 belongs to the high-risk group and has fewer chances of conception than other groups.
DISCUSSION
PCOS is diverse endocrine syndrome described by irregular menses, hyperandrogenism, and polycystic ovaries. It accounts for 90–95% of women who attend infertility clinics.10
Women with PCOS have abnormalities in the metabolism of androgens and estrogen and in the control of androgen production. High serum concentrations of androgenic hormones, such as testosterone, androstenedione, and dehydroepiandrosterone sulfate (DHEAS), may be encountered in these patients.11Also many factors affect fertility in PCOS cases .
One of the indexing factor determining the fertility potential of women is age. Age of female is an important prognostic factor in an infertile couple that affects the cumulative pregnancy rate, specifically in infertility.12 Cumulative probabilities of conception decline with age because of increased heterogeneity in fecundity in infertile couples.13 Duration of infertility and age of women are chief factors that determine subfertility.14 In this study, 76.9% of cases were ≤35 and 23.1% >35 years.
Ramlau-Hansen et al., in their Danish study, concluded that the time to pregnancy increases with increase in BMI and is associated with subfecundity.15 Among men and women with a BMI of 18.5 Kg/m2 or more, they found a dose-response relationship between increasing BMI group and subfecundity (a TTP waiting time to pregnancy of more than 12 months). In this study, more than half of our cases had a BMI of greater than 30 (51.3%). Grodstein et al. also revealed that anovulatory infertility was greater in overweight and obese patients whose BMI was found to be greater than 26.9 Kg/m2. The obese women (body mass index > or = 27) had a relative risk of ovulatory infertility of 3.1 [95% confidence interval (CI) = 2.2-4.4], compared with women of lower body weight (body mass index 20-24.9).16
Menstrual irregularity in adolescence has been shown to be a good marker of hyperandrogenaemia and it has been proposed to lead to the development of PCOS in adulthood.17
Hartz et al. established, by his study of 26,638 women, the association of obesity with infertility and menstrual abnormalities, that 12,000 women were reported to have irregular menstruation and hirsutism. Also there were 29.2% of overweight and 34% of obese women with irregular cycle.18 In this study, 69.2% had amenorrhea and 30.8% had oligomenorrhoea.
Hirusitism is a common manifestation in PCOS due to hyperandrogenemia. Alsibyani et al. in his study, conducted in 2017 in Saudi Arabian women with PCOS, reported 93.4% had hirusitism and 68.3% had oligomenorrhoea; 19 whereas, in this study only 30.8% had hirusitism.
The majority (nearly 60%) of testosterone is bound to a specific high-affinity protein called sex hormone-binding globulin (SHBG), with albumin accounting for binding to the remaining nearly 38% of the hormone. A small fraction (nearly 2% males, 1% in women) represents the physiologically active-free form that mediates the biological action of the hormone at the target tissues in both genders.20
Free androgen index (FAI) is a ratio used to determine abnormal androgen status in human. The ratio is the total testosterone level divided by the sex hormone binding globulin (SHBG) level, and then multiplying by a constant, usually 100. SHBG was low in our cases.
Regarding radiological – ultrasound findings of PCO either ovarian volume: one or two ovaries >10 cm3 or one or two ovaries >12 follicle, Alsibyani et al. in 2017 reported that 97.8% had ovarian follicles more than 2-9mm in Saudi Arabian women with PCOS.19 In this study, 84.6% had ovarian follicles more than 12 mm. Although approximately three out of four women with PCOS have polycysts on their ovaries, this clinical feature is no longer deemed necessary or sufficient for diagnosis.21
In this study, the authors identified prognostic factors for conception, calculated the total score (RII) and then categorised the infertile patients in mild, moderate and high risk groups. In this study, majority of the cases 24 (61.5%) had a moderate score of 8-10; whereas, 13 (33.3%) had severe score of >10. The higher the value of RII score, lesser are the chances for the patient to conceive; and hence, they are in the higher risk group. Hence, 33.3% of this study cases have a score greater than 10, and are in the high risk group.
Prognostic factors affect chances of pregnancy either natural or induced pregnancy.22 Although there was no significant correlation between score and outcome, majority of these cases had no pregnancy (32, 82.1%). The present authors did not find any study that calculated this score to compare with.
Regarding management of PCOS cases, Sheehan in 2004 described that generally there are but four issues such as regulation of menses, control of hirsutism, fertility issues, and the management of the insulin resistance syndrome; and its associated risks (type 2 diabetes mellitus, dyslipidemia, and cardiovascular disease).23
Risk-benefit, cost-effectiveness and patient prioritisation depend on the determination of these factors affecting fertility.24 Treatment options for infertility include clomiphene, laparoscopic ovarian drilling, gonadotropins, and assisted reproductive technology.
IVF is one of the treatments that is recommended for PCOS patients. In this study, 33.3% of cases underwent IVF, as IVF effectiveness is based on couple characteristics and focused treatment of infertile couple. Therefore, recognition and prevention of infertility associated disorder and implementing it in the care of women with PCOS and endocrine disarray should be kept into account.25 Although many studies have been conducted earlier across the world regarding diagnosis and treatment outcomes of PCOS based on certain criteria, none of those studies have been attempted to classify those outcomes into mild, moderate and high risk categories. Once the intensity of the risk of PCOS is identified, focused management can be implied there by reducing the duration and cost of the treatment. Categorisation, according to the prognostic factors, will be the new contribution to the current literature. Proper diagnosis and management of PCOS is essential to address patient concerns.10 Treatment modalities should be tracked toward focused approach by personalised decision making.22
This study was conducted in a single centre. Results from large multicenter study with considerable sample size will produce promising results to validate the RII.
CONCLUSION
Majority of the studied patients had moderate score (low risk group) than severe score (high risk group). Cases with mild score were only few. The higher the value of RII score, lesser are the chances for the patient to conceive; and hence, they are in the higher risk group. Hence, 33.3% of this study cases have a score greater than 10, and are in the high risk group. The management can be tailored for each patient according to RII score that will help in focused treatment, reduce the time period of treatment and be cost-effective.
ACKNOWLEDGEMENT:
The authors are thankful to the Research Center, King Fahad Medical City for providing the research grant and appreciate their support (IRF No. 017-042). The authors would also like to thank the Obstetrics and Gynecology Department for their kind support.
ETHICAL APPROVAL:
Study was conducted after taking approval from Reproductive Endocrinology and Infertility Medicine Department of King Fahad Medical City, Riyadh, Saudi Arabia (IRB No. 17-023).
PATIENTS' CONSENT:
Patients' consent is not applicable as this is a retrospective study for which institutional ethical board approval was taken.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS' CONTRIBUTION:
MR, DAJ: Devised the idea, performed literature searches, extracted data and wrote the protocol.
AN: Has done statistical analysis and written the interpretation of the results.
REFERENCES
- Smith S, Samantha M. Pfeifer, John A. Collins, diagnosis and management of female infertility. JAMA 2003; 290(13): 1767-70. doi: 10.1001/jama.290.13.1767.
- Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum Reprod 2007; 22(6):1506-12. doi: 10.1093/humrep/ dem046.
- Haifa A AlTurki. Prevalence of primary and secondary infertility from tertiary center in eastern Saudi Arabia. Middle East Fertility Society J 2015; 20(4):237-40. doi.org/10.1016/j.mefs.2015.02.001
- Arain F, Arif N, and Halepota H. Frequency and outcome of treatment in polycystic ovaries related infertility. Pak J Med Sci 2015; 31(3):694-99. doi: 10.12669/pjms.313.8003.
- Guraya SS. Prevalence and ultrasound features of Polycystic ovaries in young unmarried Saudi women. J Microscopy and Ultrastructure 2013; 1(1):30-4. doi.org/ 10.1016/j.jmau.2013.06.002
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 2004; 19(1):41. doi: 10.1093/humrep/deh098.
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen excess and PCOS society criteria for the polycystic ovary syndrome: The complete task force report. Fertil Steril 2009; 91(2):456-88. doi: 10.1016/j.Fertility and Sterility. 2008.06.035.
- Al-Mulhim AA, Abul-Heija AA, Al-Talib AA, Al-Turki HA, Gasim TG. Hormonal, metabolic and clinical profile of Saudi women with polycystic ovary syndrome. Saudi J Med Med Sci 2013; 1(1):30-4. DOI: 10.4103/1658-631X.112920.
- Zarchi MK, Mojaver SP, Rouhi M, Heghmatimoghaddam SH, Moghaddam NR, Anari PY, et al. Diagnostic value of risk of malignancy index for detection of pelvic malignancies compared with pathology. Electron Physician 2015; 7(7):1505-10. doi: 10.19082/1505.
- Sirmans SM, Pate KA. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 2014; 6:1-13. doi: 10.2147/CLEP.S37559.
- Badawy A, Elnashar A. Treatment options for polycystic ovary syndrome. Int J Womens Health.2011; 8(3):25-35. doi: 10.2147/IJWH.S11304.
- Collins JA, Rowe TC. Age of the female partner is a prognostic factor in prolonged unexplained infertility: A multicenter study. Fertility and Sterility 1989; 52(1):15-20. http://europepmc.org/abstract/med/2526029. doi: 10.1016/ s0015-0282(16)60781-1.
- Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Hum Reprod. 2005; 20(5):1144-7. doi: 10.1093/ humrep/deh870.
- Johannes LHE. Female subfertility. Lancet 2002; 360 (9327):151.
- Ramlau-Hansen CH, AM. Thulstrup EA, Nohr JP, Bonde TIA. Sørensen J. Olsen. Subfecundity in overweight and obese couples. Human Reproduction 2007; 22(6):1634-7. doi: 10.1093/humrep/dem035.
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology 1994; 5(2):247-50. doi:10.1016/S0140-6736(02)09417-5.
- West S, Lashen H, Bloigu A, Franks S, Puukka K, Ruokonen A, et al. Irregular menstruation and hyperandrogenaemia in adolescence are associated with polycystic ovary syndrome and infertility in later life: Northern Finland Birth Cohort 1986 study. Hum Reprod 2014; 29(10):2339-51. doi: 10.1093/humrep/deu200.
- Hartz AJ, Barboriak PN, Wong A, Katayama KP, Rimm AA. The association of obesity with infertility and related menstural abnormalities in women. Int J Obes 1979; 3(1):57‐73.
- Alsibyani NA, Malibury MA, Derham AA, Almnabri AA, Jazzar NK, Melibari RW, et al. Clinical presentation of PCOS in Jeddah-KSA. Egyptian J Hospital Medicine 2017; 67(1): 494-8.
- Al Kindi MK, Al Essry FS, Al Essry FS, Waad-Allah S Mula-Abed. Validity of serum testosterone, free androgen index, and calculated free testosterone in women with suspected hyperandrogenism. Oman Med J 2012; 27(6):471-4. doi: 10.5001/omj.2012.112.
- Dennet CC, Simon S. The role of polycystic ovary syndrome in reproductive and metabolic health: overview and approaches for treatment. Diabetes Spectr 2015; 28(2):116-20. doi: 10.2337/diaspect.28.2.116.
- McLernon DJ, te Velde ER, Steyerberg EW, Mol BWJ, Bhattacharya S. Clinical prediction models to inform individualized decision-making in subfertile couples: A stratified medicine approach. Hum Reprod 2014; 29(9): 1851-8. doi: 10.1093/humrep/deu173.
- Sheehan MT. Polycystic ovarian syndrome: Diagnosis and management. Clin Med Res 2004; 2(1):13-27.
- Messerlian C, Jennifer R. Fishman. Less is more: Improving outcomes and cutting costs to Quebec’s assisted reproduction program. CMAJ 2014; 186(6):405-6. doi: 10.1503/cmaj.131672.
- Meniru GI, Bryan R. Hecht, Michael P. Comprehensive preventive healthcare in female-factor infertility: Issues, problems and proposals. Primary Care Update 2002; 9(1):44-50. doi:10.1016/S1068-607X(01)00102-0.