Right to Left Intrapulmonary Shunt in a Case with COVID-19-associated Pneumonia
By Maimoona Siddique1, Muhammad Khalid Nawaz1, Muhammad Bin Khalid1, Aitzaz Rai2Affiliations
doi: 10.29271/jcpsp.2021.01.23ABSTRACT
The clinical manifestations of coronavirus disease 2019 (COVID-19)-associated pneumonia show a wide range of variations. It ranges from mild hypoxemia without significant signs of respiratory distress, to rapid clinically deteriorating course with severe hypoxemia. Unexplained severe hypoxemia, associated with platypnea, triggers the possibility of ventilation-perfusion (V/Q) mismatch, ranging from intrapulmonary shunts (IPS) to alveolar dead space ventilation. In the literature, very few cases with COVID-19-pneumonia have been reported with IPS.
Herein, we report a COVID-19 confirmed 45-year male patient, who developed IPS without apparent pulmonary perfusion defect on lung perfusion scintigraphy. The patient had no cardiovascular disease except chronic pulmonary hypertension secondary to interstitial lung disease. The clinical manifestations combined with nuclear imaging features enabled in making the ultimate diagnosis. The patient’s clinical condition improved on appropriate clinical management, using high flow oxygen combined with intravenous steroids and anticoagulants.
Key Words: COVID-19, Adult respiratory distress syndrome, Right to left shunt, Lung perfusion scintigraphy, Platypnea.
INTRODUCTION
Originating from Wuhan city of China in December 2019, a novel beta‐coronavirus, known as severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), was identified as the coronavirus disease 2019 (COVID-19) pathogen. The pandemic has alarmingly raised many challenges in the intensive care management of the COVID-19-related acute respiratory distress syndrome (C-ARDS).1
C-ARDS may present with unexplained hypoxemia, leading to platypnea and falling oxygen saturation in the upright position. It may or may not show radiological evidence of pulmonary embolism.2 COVID-19 may induce a thrombogenic phenomenon and should be considered in those cases with unexplained hypoxemia, despite the absence of apparent cardiogenic risk factors.3
Nuclear lung perfusion imaging for the right to left shunt quantification is one of the mainstays of diagnostic imaging modalities.
We present a COVID-19 infected case with no evidence of pulmonary embolism on diagnostic computed tomography pulmonary angiography (CTPA). The patient had severe platypnea and a lung perfusion scan confirmed the clinical suspicion of right to left shunting.
CASE REPORT
A 45-year male presented with a history of high-grade fever and progressive dyspnea. The real-time polymerase chain reaction (RT-PCR) was positive for COVID-19.
The patient had falling saturation in an upright position that improved on lying flat in bed. The transthoracic echocardiogram (TTE) showed evidence of pulmonary hypertension, without signs of heart failure; and a bubble study, raised the concern for intrapulmonary shunt (IPS). Arterial blood gas measurements repeatedly revealed hypoxemia with oxygen saturation in the range of 80% to 90%.
Figure 1 shows the serial axial CT pulmonary angiograms (CTPAs). The patient had progressively worsening platypnea with falling oxygen saturation (70-90%) despite high flow oxygen (15 L/min). Laboratory results showed raised C-reactive protein and D-dimers, with a high neutrophil-lymphocyte ratio at 1:16. The subsequent CTPA performed on day-8 and 14, showed radiological evidence of COVID-19-associated vasculopathy.
To rule out the clinical suspicion of IPS secondary to pulmonary infection, the patient underwent Tc-99m macro-aggregated albumin (MAA) lung perfusion scan after the intravenous junction of 5 mCi of Tc-99m MAA, as shown in Figure 2.
The patient was treated with oxygen therapy, steroids, antibiotics, and heparin. On day-21, the patient was discharged with instructions for warfarin and oxygen therapy.
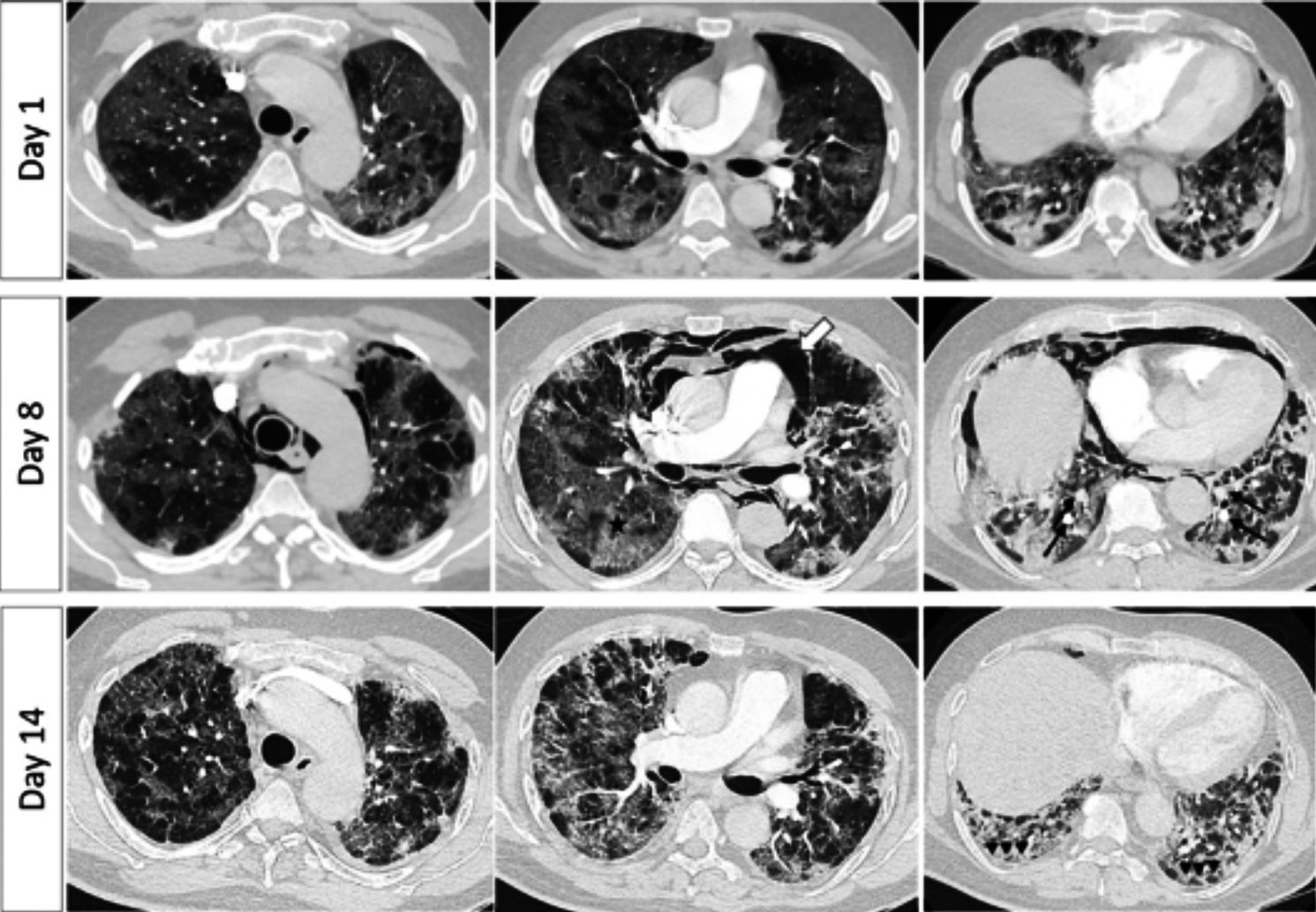 Figure 1: Day 1 axial CTPA lung window images (Top row) show patchy ground-glass opacities in both lungs’ periphery and bases, with no evidence of pulmonary embolism (PE). Serial CTPAs performed on day-8 and 14, showed dilated pulmonary vessels in the lung bases (marked arrows) with interlobular septal thickening, crazy paving segmental/subsegmental consolidation. There was pneumo-mediastinum (thick arrow) that resolved later on. The subpleural subsegmental pulmonary vessels were more tortuous posteriorly (arrowheads) with olegemic lungs anteriorly, suggestive of COVID-19-associated vasculopathy/ARD.
Figure 1: Day 1 axial CTPA lung window images (Top row) show patchy ground-glass opacities in both lungs’ periphery and bases, with no evidence of pulmonary embolism (PE). Serial CTPAs performed on day-8 and 14, showed dilated pulmonary vessels in the lung bases (marked arrows) with interlobular septal thickening, crazy paving segmental/subsegmental consolidation. There was pneumo-mediastinum (thick arrow) that resolved later on. The subpleural subsegmental pulmonary vessels were more tortuous posteriorly (arrowheads) with olegemic lungs anteriorly, suggestive of COVID-19-associated vasculopathy/ARD.
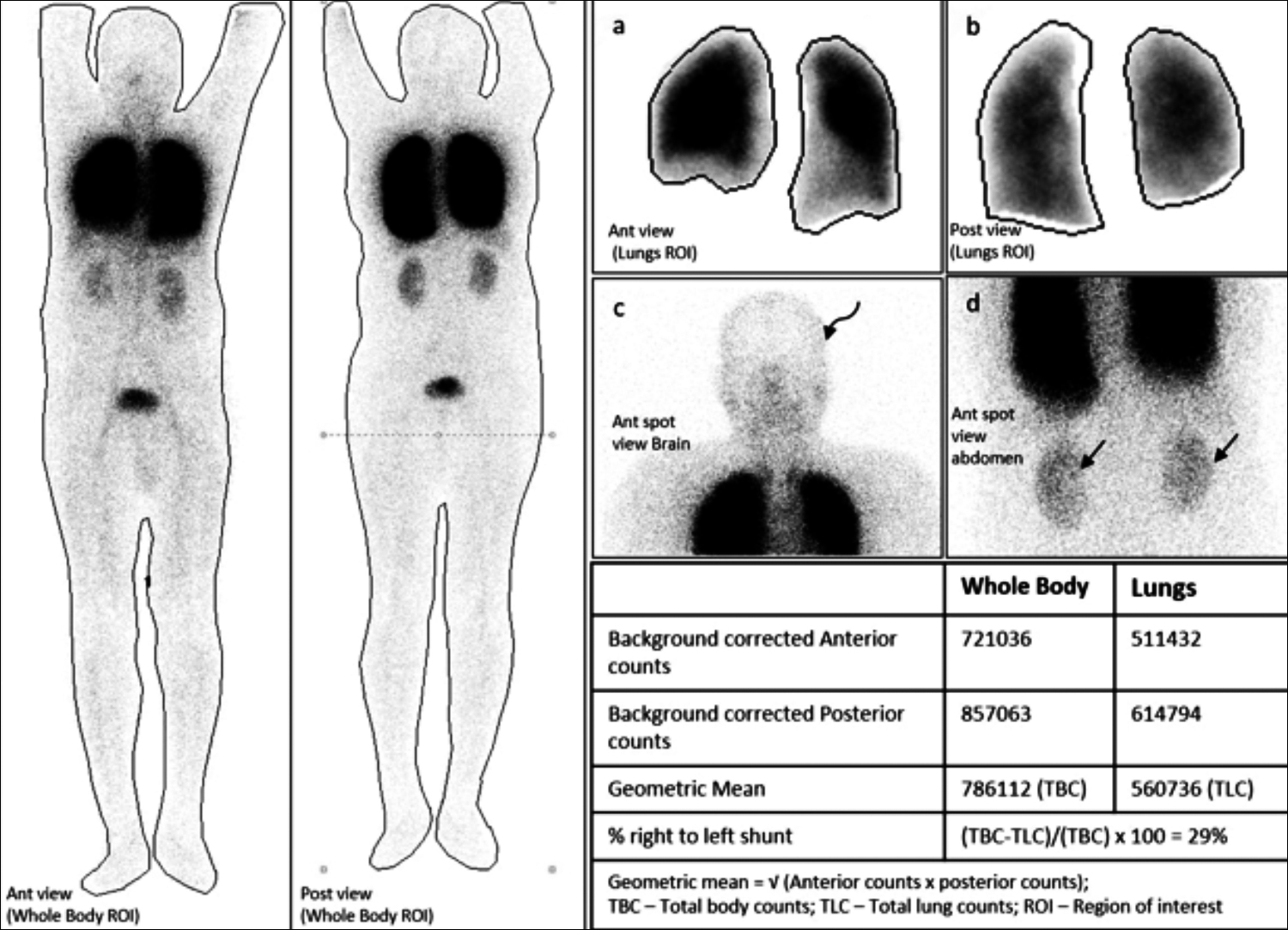 Figure 2: Lung perfusion static images show mild tracer uptake in the brain (curved arrow, c) and kidneys (straight arrows, d). The quantitative analysis estimated a lung shunt fraction of 29% (reference value <7%), suggestive of right-to-left shunt. No segmental/subsegmental pulmonary perfusion defect was seen, indicating a low probability of pulmonary embolism.
Figure 2: Lung perfusion static images show mild tracer uptake in the brain (curved arrow, c) and kidneys (straight arrows, d). The quantitative analysis estimated a lung shunt fraction of 29% (reference value <7%), suggestive of right-to-left shunt. No segmental/subsegmental pulmonary perfusion defect was seen, indicating a low probability of pulmonary embolism.
DISCUSSION
With the rapid surge and heterogeneity of C-ARDS cases worldwide, the medical profession is putting its efforts to describe the subgroups of patients with different clinical and biological characteristics and treatment responses.3
Apart from hypoxemia, in rare COVID-19 pneumonia cases, IPS may occur in perfused non-aerated lung areas secondary to pulmonary vessel dilatation, without intracardiac shunt on bubble TTE.4 In a study, Masi P et al.5 detected IPS in 12 out of 60 critically ill patients with confirmed C-ARDS on bubble TTE. It emphasises the concern for looking into the possibility of a pulmonary shunt in cases with unexplained hypoxemia that gets exacerbated in an upright position, a condition known as platypnea-orthodeoxia syndrome. The possible causes of this syndrome are intra-cardiac shunt, pulmonary parenchymal V/Q mismatch, idiopathic pulmonary hypertension, long-standing lung disease, and pulmonary arteriovenous shunts, leading to the transient right to left shunt.6
On lung perfusion scintigraphy, extra-pulmonary accumulation of MAA occurs when the tracer bypasses the lungs due to right to left cardiac or pulmonary shunt. The abnormal tracer uptake on pulmonary perfusion scintigraphy in brain, kidneys, liver and spleen without free pertechnetate in the thyroid is typical of right to left shunt.
Right-to-left pulmonary shunt due to microscopic angiopathy is a rare manifestation of C-ARDS. Lung perfusion nuclear imaging confirms this diagnosis noninvasively.
PATIENT'S CONSENT:
Informed consents was obtained from patient to publish the data concerning this case.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
MS: Collected the data and wrote the manuscript.
KN: Critically reviewed the write-up and edited the final manuscript.
MBK: Contributed to prepare the figure legends.
AR: Reviewed the final manuscript and modified it according to the edits suggested by the reviewers.
All the authors have agreed to the modifications made in the final draft.
REFERENCES
- Li X, Ma X. Acute respiratory failure in COVID-19: Is it “typical” ARDS? Crit Care 2020; 24(1):198. DOI: 10.1186/ s13054-020-02911-9.
- Fan E, Beitler JR, Brochard L, Calfee CS, Furgosun ND, Slutsky AS, et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Resp Med 2020; 8(8):816-21. DOI: 10.1016/ S2213-2600(20)30304-0.
- Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med 2020; 46(6):1099-1102. DOI: 10.1007/s00134-020- 06033-2.
- Boissier F, Razazi K, Thille AW, Roche-Campo F, Leon R, Vivier E, et al. Echocardiographic detection of transpulmonary bubble transit during acute respiratory distress syndrome. Ann Intensive Care 2015; 5:5. DOI: 10.1186/s13613-015-0046-z.
- Masi P, Bagate F, d’Humières T, Al-Assaad L, Chakra LA, Derumeaux G, et al. Is hypoxemia explained by intracardiac or intrapulmonary shunt in COVID-19-related acute respiratory distress syndrome? Ann Intensive Care 2020; 10(1):108. DOI: 10.1186/s13613-020-00726-z.
- Moses CA and Mishkin FS. Right-to-left shunt on a lung scan. N Engl J Med 1999; 340(11):857. DOI: 10.1056/NEJM 199903183401106.