Restenosis after Balloon Dilatation with versus without Stent Placement for Hepatic Vein Involvement Budd-Chiari Syndrome
By Xuedong Sun, Lulin Zhou, Maoqiang WangAffiliations
doi: 10.29271/jcpsp.2021.04.455ABSTRACT
Balloon angioplasty with or without stent placement has become the mainstream treatment of Budd–Chiari syndrome (BCS). Restenosis of hepatic vein (HV) is a tough problem. The aim of this study was to perform a meta-analysis to compare the restenosis in HV involvement type BCS patients treated by balloon dilatation with versus without stent. Meta-analysis was used to calculate the combined effect size and their 95% confidence intervals (CI), based on random effect; and calculate the risk ratio (RR) and its 95% CI based on fixed effect. The publication bias was assessed by funnel plot and Begg’s test. Sixteen studies were selected for meta-analysis. One thousand and eighty-two patients (1,019 from Asian, 63 from non-Asian countries) were included. Seven hundred and five of 1,019 (69%) Asian patients received HV balloon dilatation alone. RR value (RR=0.85, 95% CI 0.68-1.08) of the two groups was obtained through meta-analysis, which meant that the risk of restenosis in balloon dilatation alone group was 15% lower than that in combined with stent placement group; although there was no significant statistical difference between two groups (p=0.178). The current meta-analysis indicated that balloon dilatation alone is first preferred approach in Asian BCS patients compared to the non-Asian patients. Balloon dilatation combined with stent placement does not reduce restenosis risk over balloon dilation alone in the treatment of BCS with HV occlusion. It is suggested that stent should be used cautiously for such patients.
Key Words: Balloon dilatation, Budd-Chiari syndrome, Stent, Restenosis, Meta-analysis.
INTRODUCTION
In 1845, British clinician William Budd described the abnormally thickened hepatic veins (HVs) for the first time in his seminal book "On diseases of the liver", considering HV occlusion caused by HV inflammation.1 In 1899, the Austrian pathologist Hans Chiari reported 13 cases of venous endometritis at the opening of the HV, which was called HV occlusive phlebitis.2 This liver vascular disorder was called BCS.
In China, the majority of HV involvement BCS were membranous obstructive lesions, accounting for about 88-99%.3,4 Therefore, most patients could obtain good curative effect by HV recanalisation. In western countries, only 25%-41% of patients were membranous or short segment (< 4cm) lesions of proximal HV and the majority were diffuse obstructive lesions of HVs. Therefore, transjugular intrahepatic portosystemic shunt (TIPS) was often used.5,6
Scholars had different opinions on whether to implant stent after balloon dilatation.7 A recently published prospective randomised controlled study suggested that routine stent implantation could reduce the risk of restenosis in patients with BCS. However, only 11 HV type BCS patients were included in this study, and there was no comparative analysis according to the lesion types, so it is insufficient evidence in evidence-based medicine.8
Therefore, this meta-analysis was conducted to quantitatively study the restenosis rate of balloon dilatation with or without stent placement, in order to provide the basis for clinical treatment of BCS with HV occlusion.
METHODOLOGY
CNKI, Wanfang, VIP, PubMed, Cochrane Library and EMBASE databases were searched from the establishment of the database to May 5, 2020. Clinical studies of HV recanalisation in patients with BCS with HV obstruction were collected. In addition, the references of the included literature were also reviewed to obtain more relevant literature as far as possible. The subject words and free words were used for retrieval. The main search terms include Budd-Chiari syndrome, hepatic vein, hepatic vein obstruction, hepatic venous outflow block, radiological intervention, recanalisation, endovascular treatment, venoplasty, balloon dilatation, balloon angioplasty, and stent.
Each searched study was separately reviewed by two researchers (two interventional radiologists with experience of 13 and 9 years, respectively) to determine if it was suitable for inclusion. The inclusion criteria were: the participants diagnosed with primary BCS with HV obstruction and treated by HV recanalisation, observational studies regardless of retrospective or prospective data, and detailed information of treatment plan, restenosis cases, etc. If studies involving the same population or from the same medical team had been published for many times, the study with the largest sample size, the longest inclusion period or the latest published study was selected; publication date, publication language, or publication status were not restricted. Exclusion criteria were: non-availability of full text of the literature, incomplete and unclear data, inconsistent outcome indicators, case reports (<10 patients), nursing related, surgical treatment, comment, review, diagnostic, dissertation, conference paper, animal studies, etc. If the two researchers did not reach an agreement about eligibility of the article, the third reviewer participated in consensus building.
Data were extracted by two reviewers independently and disagreements were adjudicated by the third interventional radiologist with experience of 30 years. Data were collected from all 16 studies regarding first author, publication year, country, demographic data (age), study duration, follow-up duration, the time of primary restenosis, study results (the number of patients who underwent HV balloon dilatation and HV stent placement, and the number of patients with primary restenosis after HV recanalization in each group).
Based on the sample size of individual studies, exact binomial confidence intervals were calculated for each study, and pooled measure was calculated as along with an equal weighted average rate (weighted by each study’s sample size) in all samples by forest plot. The I2 index were used to assess heterogeneity among studies. The ‘leave one out’ sensitive analysis was carried out using I2 >50 % and p <0.1 as the criteria to evaluate the studies with substantial impact on between-study heterogeneity. Publication bias was estimated by Begg’s test and funnel plots. All statistical analyses were performed with STATA version 15 (Stata Corporation, College Station, Texas, USA). All reported probabilities (p values) were two-sided, with p < 0.05 considered statistically significant.
RESULTS
The study identification and selection processes were shown in Figure 1. Sixteen eligible studies which conformed the inclusion criteria, included 1,082 (1019 from Asian, 63 from non-Asian countries) BCS with HV obstruction patients treated by HV recanalisation in this meta-analysis. Among them, 737 (68%) cases (705 from Asian, 32 from non-Asian patients) underwent balloon dilatation alone, 345 (32%) cases (314 from Asian, 31 from non-Asian patients) underwent balloon dilatation with stent placement. A total of 280 cases (26%) developed restenosis, 188 cases occurred in balloon dilatation alone group and 92 cases in balloon dilatation with stent placement group. Eleven studies were from China, four from India, and one from England. Two studies were reported in Chinese language, the remaining 14 were in English. Baseline characteristics of included studies were shown in Table I.
Restenosis was defined as no or reverse flow signals in the original opened HV or accessory HV lumen detected by ultrasonography, or the stenosis was more than 30% of the original lumen diameter after venoplasty, and collateral vessels were formed simultaneously, with or without recurrence of BCS related symptoms. PTBA was defined as balloon dilatation alone. Stent was defined as balloon dilatation combined with stent placement.
Fourteen papers were analysed for this meta-analysis. Heterogeneity test (I2=0.0 % <50%, p=0.83 >0.1) suggested that there was no significant heterogeneity among the included 14 literatures. Based on the fixed effect, the RR value (RR=0.85, 95%CI 0.68-1.08, p=0.178) of the two groups was obtained (Figure 2). In conclusion, the risk of restenosis in PTBA group was 15%, lower than that in stent group, although there was no significant statistical difference between two groups.
Fourteen papers were analysed for this meta-analysis. Heterogeneity test (I2=70.03% >50%, p <0.01) suggested that there was significant heterogeneity between the included 14 literatures. In order to ensure the accuracy and stability of the study, sensitivity analysis was conducted. Sensitivity analysis showed none of 14 literatures caused great interference to the results of this meta-analysis, indicating that this study has good stability. Based on the meta-analysis of random effect, the total effect quantity of 14 studies was 0.21, and the 95% confidence interval was 0.13-0.29, which was statistically significant (p <0.001, Figure 3). The pooled result (95 % CI) of the restenosis rate in PTBA group was 21% (13-29%). Sixteen papers were analysed for this meta-analysis. Heterogeneity test (I2=49.25% <50%, p <0.1) suggested that there was significant heterogeneity between the included 16 literatures. In order to ensure the accuracy and stability of the study, sensitivity analysis was conducted. Sensitivity analysis showed none of 16 literatures caused great interference to the results of this meta-analysis, indicating that this study has good stability. Based on the meta-analysis of random effect, the total effect quantity of 16 studies was 0.21, and the 95% confidence interval was 0.13-0.29, which was statistically significant (p <0.001, Figure 4). The pooled result (95 % CI) of the restenosis rate in stent group was 21% (13-29%).
Funnel plot was used to detect publication bias in comparison of restenosis rate between the two groups. The results showed that both sides of funnel plot were basically symmetrical (Figure 5), indicating no obvious publication bias. Begg’s test was further applied to assess publication bias of the results in the studies. It indicated that there was no publication bias on the restenosis rate of PTBA group (p=0.701), stent group (p=0.300), PTBA versus stent group (p=0.913).
Table I: Baseline characteristics of included studies of interventional treatment with HV involvement type BCS.|
First author (year of publication) |
Country |
Study type |
Study duration |
Age |
Interventional treatment, NO |
Restenosis, NO |
Time of restenosis post interventional treatment, M |
Follow-up duration, M |
|||
|
PTBA |
Stent |
PTBA |
Stent |
PTBA |
Stent |
||||||
|
Li Dongmei |
China |
cohort study |
2011.1-2018.12 |
36.1±11.1 |
40 |
6 |
10 |
2 |
NA |
NA |
30.3±18.3(0.1-96) |
|
Wang Qiuhe |
China |
RCT |
2014.7-2017.9 |
NA |
6 |
5 |
2 |
0 |
NA |
NA |
27(19-41) |
|
Chen Zhongke |
China |
cohort study |
2011.6-2016.8 |
32.6 ± 10.8 |
60 |
8 |
17 |
2 |
NA |
NA |
29.4 ± 13.6 |
|
Amar Mukund |
India |
cohort study |
2010.11-2014.10 |
31.6 (1–67) |
14 |
48 |
0 |
4 |
None |
0.25,3,12,8 |
43(0-72) |
|
Zu Maoheng |
China |
cohort study |
1990.1-2017.5 |
43.3 (2-79) |
361 |
106 |
99 |
41 |
NA |
NA |
NA |
|
Singh |
India |
cohort study |
2000.4-2016.8 |
10.5 (2-17) |
4 |
23 |
0 |
8 |
None |
NA |
13.5 (1-155) |
|
Tripathi |
England |
cohort study |
1987-2014 |
34.9 ± 10.9 |
32 |
31 |
12 |
6 |
0.6-78 |
0.5-17 |
113(1.2-1178.6) |
|
Fan Xinxin |
China |
cohort study |
1995.5-2014.12 |
38.82±11.45 |
0 |
27 |
0 |
3 |
None |
NA |
82.25±46.16 |
|
Ding Pengxu |
China |
cohort study |
2005.1-2013.12 |
39.83±12.54 (15-72) |
90 |
0 |
8 |
0 |
NA |
None |
52.46±27.99(1-96) |
|
Sang Hongfei |
China |
cohort study |
2003.6-2012.6 |
42±2.2 (17–71) |
15 |
3 |
24 |
5 |
NA |
NA |
24 ± 1.3(6–62) |
|
Kathuria |
India |
cohort study |
2000.1-2011.2 |
10.5 (2–16) |
3 |
18 |
1 |
4 |
13 |
0.5,14,75.6 |
6.5 |
|
Zhang Bo |
China |
cohort study |
2007.6-2012.6 |
36±9 (19-50) |
0 |
15 |
0 |
3 |
None |
0.5,4,21 |
27.6±15.4(6-61) |
|
Ahmed Eldorry |
India |
cohort study |
NA |
28.28 ± 8.93 (14-57) |
2 |
10 |
1 |
4 |
0.25 |
0.25,1,3,4 |
27.6±15.4(6-61) |
|
Li Tianxiao |
China |
cohort study |
1996.9-2008.10 |
31.3 (15-57) |
90 |
2 |
28 |
0 |
<24 |
None |
24 |
|
Yu Chaowen |
China |
cohort study |
2004-2008 |
19-35 |
14 |
2 |
1 |
0 |
12 |
None |
3-38 |
|
Li Zhengran |
China |
cohort study |
1998-2003 |
42 (24-63) |
6 |
18 |
2 |
5 |
<3 |
<24 |
3-24 |
|
Data are presented as n = mean; standard deviation = (± SD); median, or range; BCS = Budd-Chiari syndrome; HV = Hepatic vein; PTBA = percutaneous transluminal balloon angioplasty; RCT = randomised controlled study; No. = number; M = months, NA = not available. |
|||||||||||
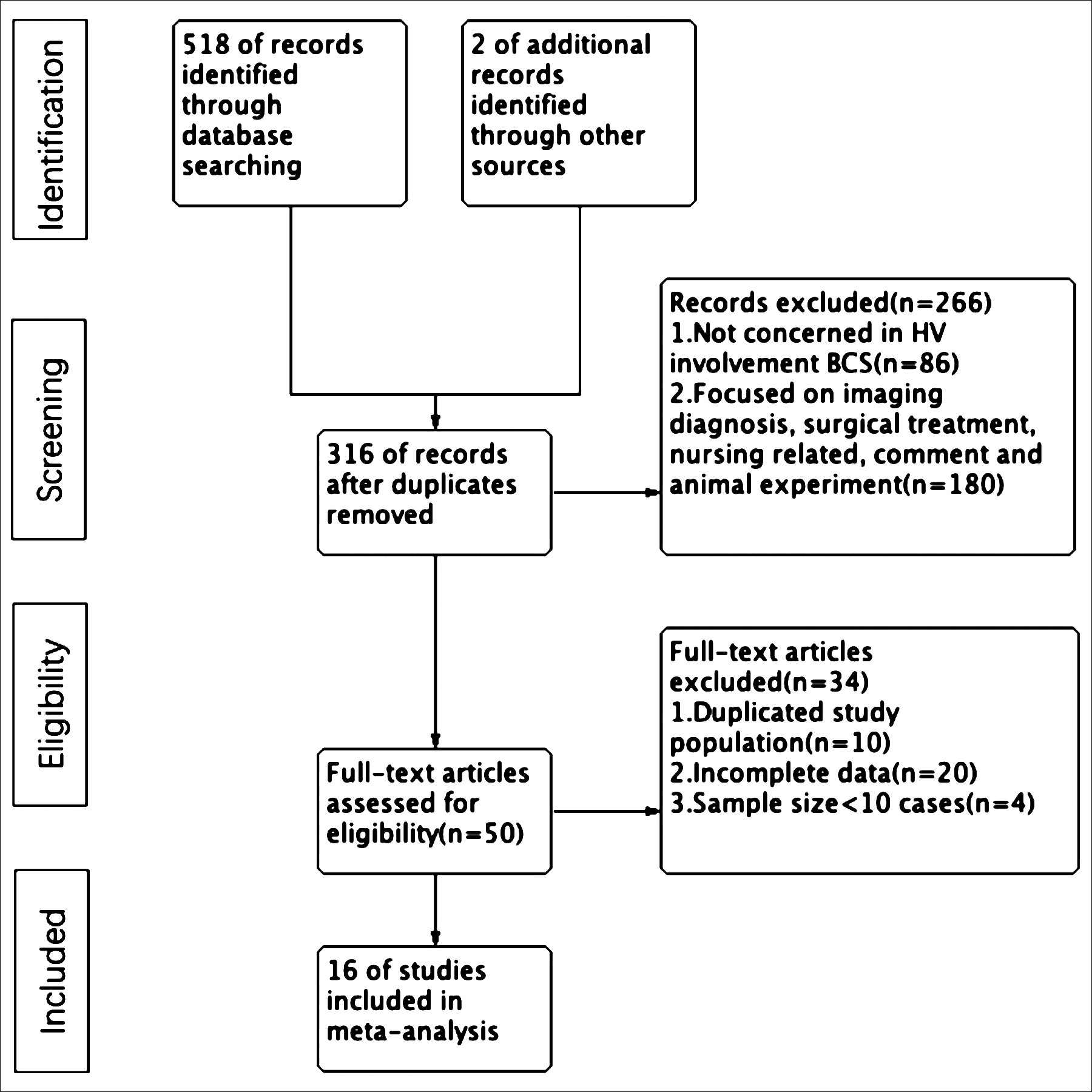 Figure 1: Flow diagram of studies selection in meta-analysis. BCS, Budd–Chiari syndrome, HV, hepatic vein.
Figure 1: Flow diagram of studies selection in meta-analysis. BCS, Budd–Chiari syndrome, HV, hepatic vein.
DISCUSSION
To the authors’ knowledge, the present study was the first meta-analysis to evaluate the restenosis rate in HV involvement type BCS, treated with balloon dilatation with versus without stent. The findings from this meta-analysis indicated that there was no significant difference in restenosis rate between balloon dilatation alone, and balloon dilatation combined with stent placement. It is suggested that balloon dilatation alone is first preferred approach in Asian BCS patients compared to the non-Asian. Stent placement is not supported as the first-line treatment, but only as a supplementary treatment for obvious residual stenosis after balloon dilatation.
A total of 16 studies were included in this meta-analysis, 11 from China, four from India and one from England. It indicated that the incidence rate of BCS in Asia was relatively high. In China, HV lesions were mostly membranous or short segmental obstructive lesions, so the first choice was HV recanalisation.3
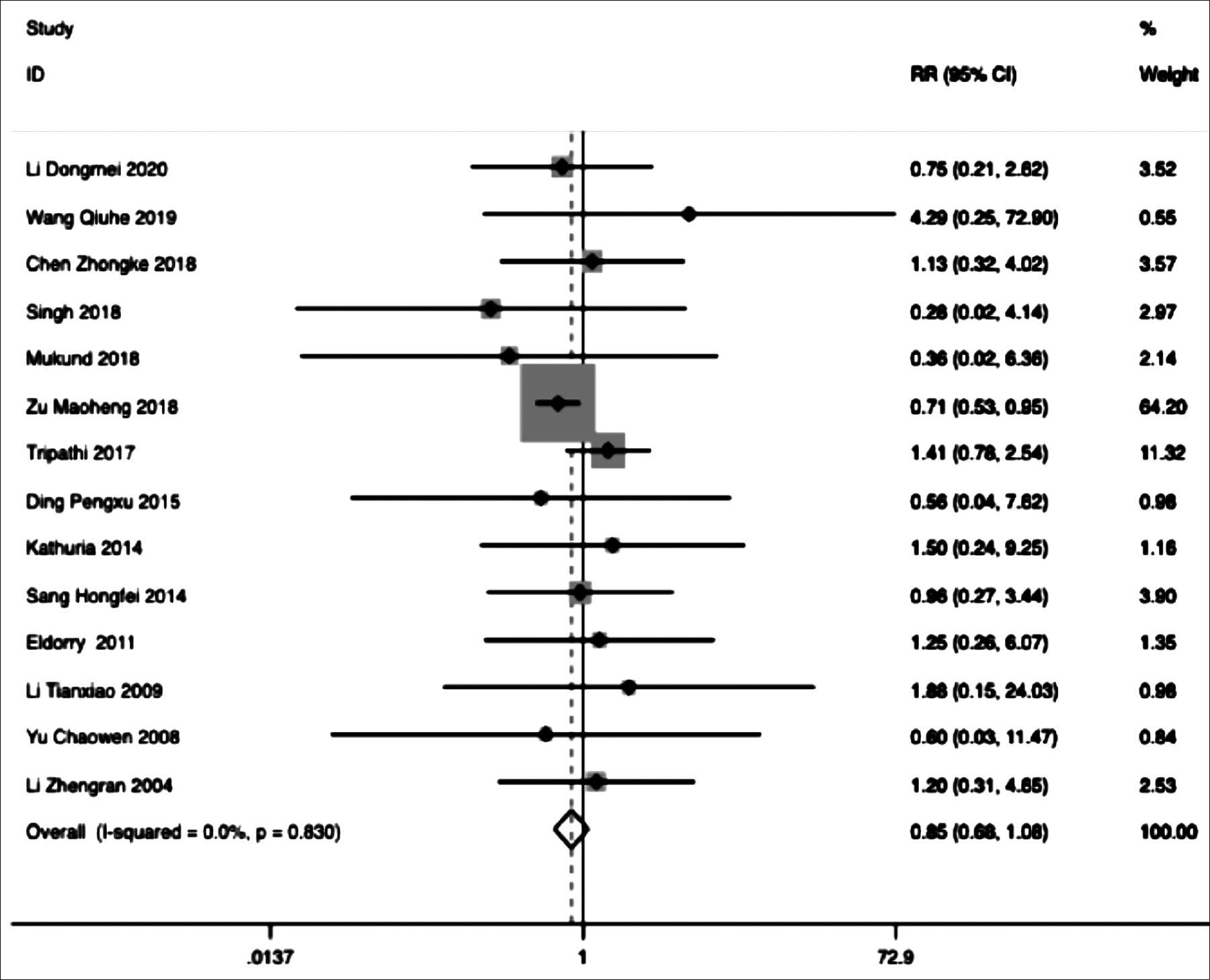 Figure 2: Forest plot of the restenosis rate of PTBA versus stent. RR, risk ratio.
Figure 2: Forest plot of the restenosis rate of PTBA versus stent. RR, risk ratio.
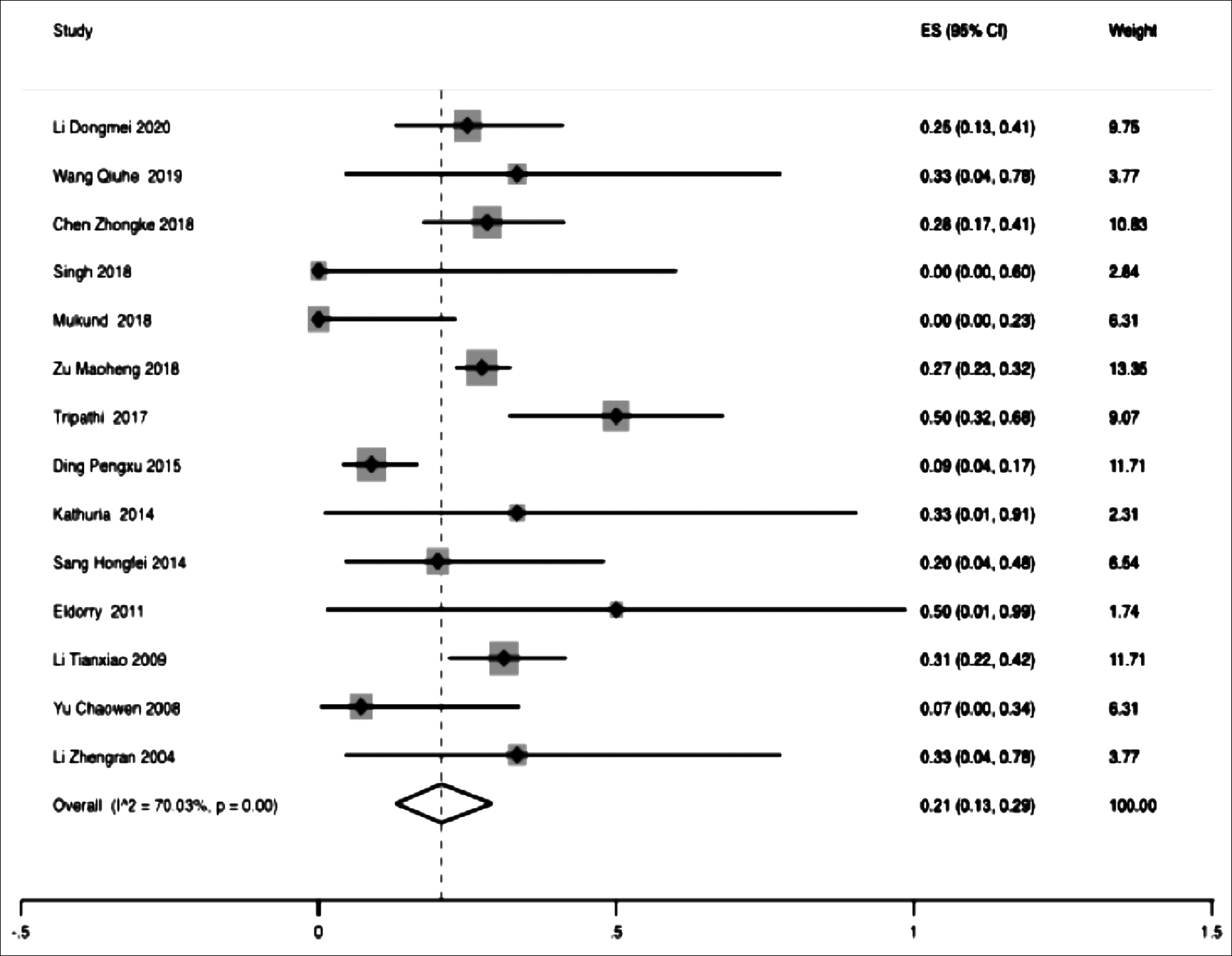 Figure 3: Forest plot of the restenosis rate of PTBA group. ES, effect size.
Figure 3: Forest plot of the restenosis rate of PTBA group. ES, effect size.
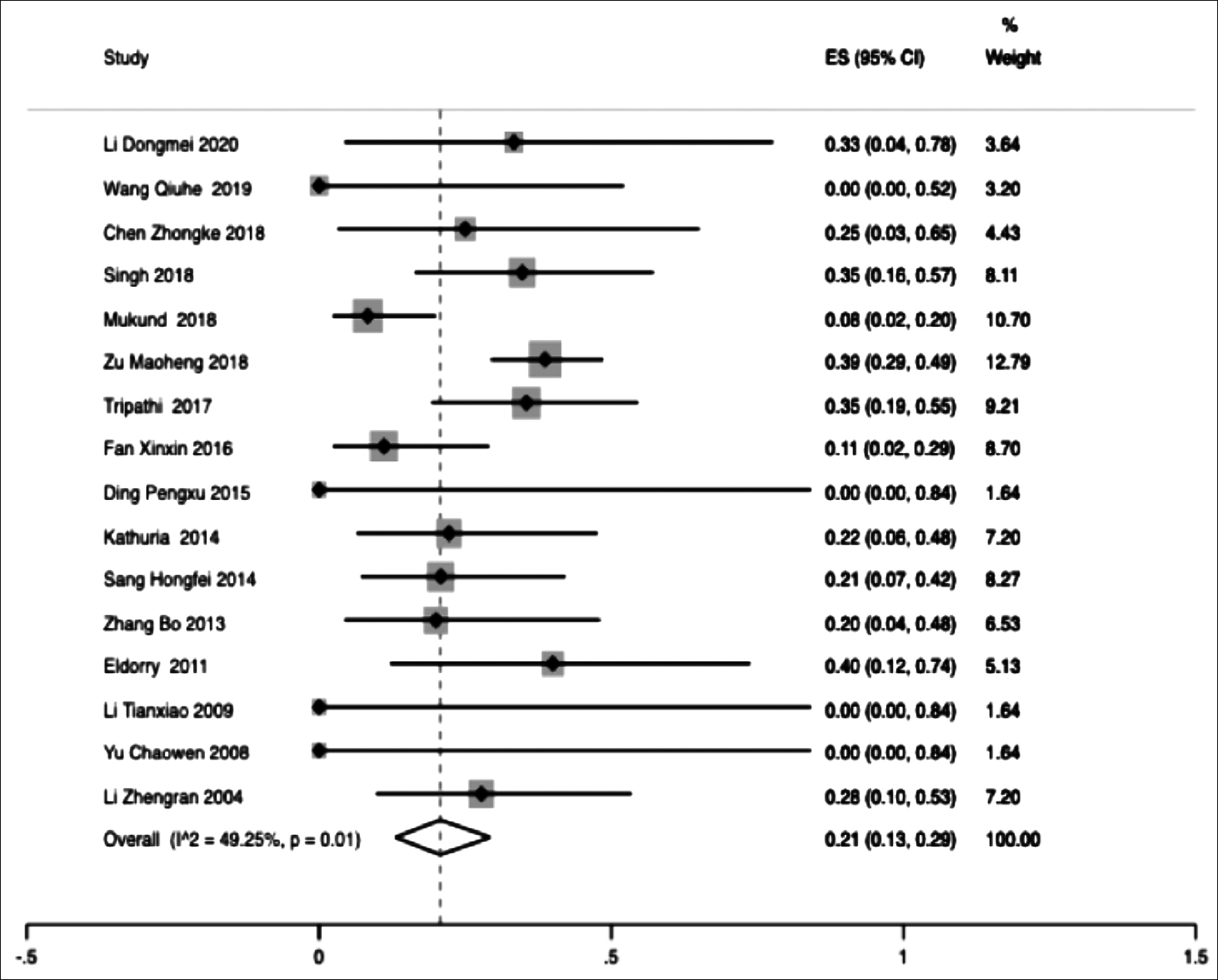 Figure 4: Forest plot of the restenosis rate of stent group; ES, effect size.
Figure 4: Forest plot of the restenosis rate of stent group; ES, effect size.
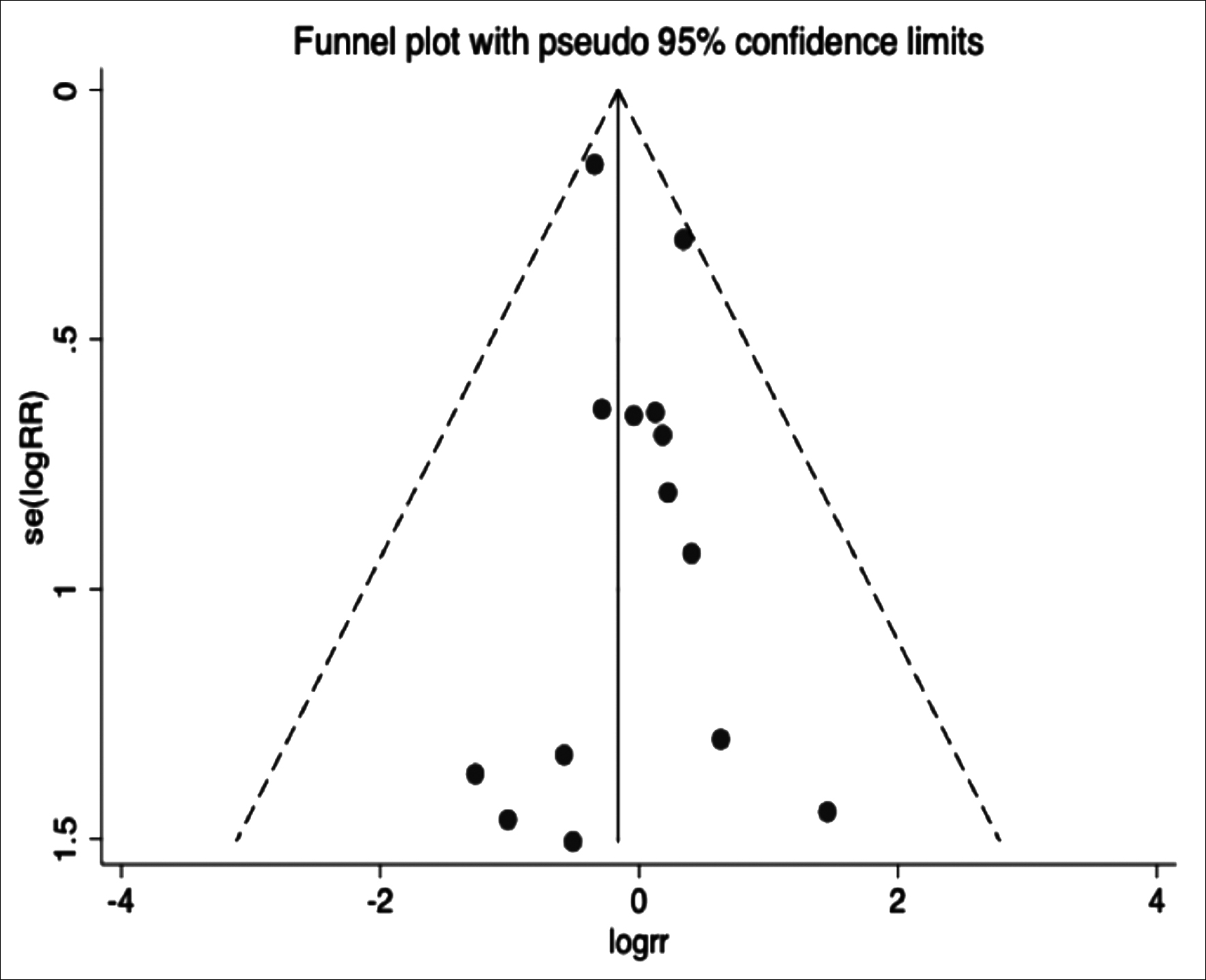 Figure 5: Funnel plot of the restenosis rate of PTBA versus stent.
Figure 5: Funnel plot of the restenosis rate of PTBA versus stent.
When HV recanalisation was not successful or the symptoms were not relieved after venoplasty or there was irreversible severe cirrhosis with serious complications (such as repeated esophageal varices, upper gastrointestinal bleeding, intractable ascites), TIPS should be performed in time.9 However, in Europe, diffuse occlusion of HVs was common, and HV short segment obstructive lesion accounted for only 25%.5,6 Therefore, majority chose TIPS instead of HV recanalisation in Europe.10-12 A total of 1,082 patients were enrolled in this study. All patients underwent HV recanalisation due to membranous or segmental HV occlusion. Among them, 737(68%) cases (705 from Asian, 32 from non-Asian) underwent HV balloon dilatation alone, 345(32%) cases underwent balloon dilatation combined with stent implantation, which indicated that balloon dilatation alone is still the mainstream treatment for HV involvement BCS, especially in Asian countries (69%, 705/1019). Restenosis occurred in 280 cases (26%). Restenosis leads to disturbance of hepatic blood flow, degeneration and necrosis of hepatocytes around the centre of hepatic lobule, which leads to the changes of laboratory indexes such as liver function. Meanwhile, intrahepatic fibrous tissue hyperplasia, portal vein pressure increase, gastrointestinal bleeding and intractable ascites are produced. A 30-year follow-up study by Zhang et al. suggested that restenosis was an independent factor for survival of BCS patients after interventional therapy.13 Therefore, the occurrence of restenosis is the core factor affecting the survival. Timely detection of restenosis and re-intervention is the key to improve the prognosis.
The problem of restenosis after BCS intervention treatment has been plaguing clinicians. Restenosis is often accompanied by recurrence of symptoms, which means that the previous treatment failed, and patients need to undergo re-intervention or other treatments, which increases the patient's pain and financial burden. In Europe, European Association for the Study of the Liver guidelines suggested that HV stent placement can reduce restenosis,7 but there is no specific clinical data to support it. In China, interventional therapy experts generally recommend that the indication of HV stent placement is that the pressure drop of HV after balloon dilatation is not ideal, or the elastic retraction of HV lumen is more than 50%.14
In this study, the restenosis rate after balloon dilatation alone and balloon dilatation combined with stent implantation was compared by meta-analysis. The results showed that the incidence of restenosis in balloon dilatation alone group was 85%; of that in balloon dilatation combined with stent placement group, that is, the risk of restenosis was 15% lower than that of stent placement, but there was no significant difference (RR = 0.85, 95% CI 0.68-1.08, P = 0.178). Although it is not certain that balloon dilatation alone has a lower incidence of restenosis than balloon dilatation combined with stent placement, it can be confirmed that balloon dilatation combined with stent placement does not show obvious advantages in reducing postoperative restenosis compared with balloon dilatation alone. Tripathi et al. retrospectively analysed 63 HV involvement BCS patients who underwent angioplasty in a single centre for 27 years, and the median follow-up time was 113.0 months.15 In the balloon dilation group, 16 cases (50%) occurred restenosis; in the balloon dilatation combined with stent placement group, 11 cases (35.5%) occurred restenosis, but we did not know whether there was any statistical difference between the two groups. Cui et al. retrospectively analysed 140 patients with HV involvement BCS who received HV recanalisation successfully.4 The average follow-up time was 33.9 ± 15.3 months (7-75 months). There were 22 cases (18.3%) in PTBA alone group and 6 cases (3.8%) in PTBA with stent group occurred restenosis, but there was no significant difference between two groups. It also suggested that HV segmental occlusion is an independent predictor of restenosis.4 Zu et al. reported the average time of the restenosis occurrence was 23.65 ± 2.60 months.16 Compared with balloon dilatation alone, stent placement increases more risks. Zu et al. retrospectively analysed 49 cases of patients with HV stent implantation. Two cases (4.1%) had IVC obstruction, and five cases (2%) had stent rupture.17 The anatomic characteristics of HV are gradually thinning from proximal to distal. Clinical available HV stents are straight tube. The distal end of stent is easy to aggravate and stimulate intimal hyperplasia. Therefore, restenosis at the distal end of HV stent is common. If the stent re-obstruction, it will bring difficulties for re-intervention, or lose the opportunity to open the HV again. Therefore, stent placement should be cautiously selected for HV involvement BCS patients.
This study has few limitations. Firstly, the operational proficiency and the choice of instruments influenced the treatment outcomes. Secondly, most of the literatures in this meta-analysis are single arm studies, and part of the heterogeneity comes from this. Thirdly, heterogeneity may also be due to differences in research methods, including selection bias. When the RR values between PTBA and stent group through meta-analysis were calculated, there was no heterogeneity. Heterogeneity appeared in the calculation of the combined effect of each group, but sensitivity analysis showed that none of the inclusive literatures caused great interference to the results of this meta-analysis, which means that these studies have good stability. Begg’s test for each group also found no publication bias.
CONCLUSION
Findings from this meta-analysis indicate that balloon dilatation alone is first preferred approach in Asian BCS patients compared to the non-Asian. Balloon dilatation combined with stent implantation does not show obvious advantages over balloon dilation alone in the treatment of BCS with HV occlusion. Stent implantation is not supported as the first-line treatment, but only as a supplementary treatment for obvious residual stenosis after balloon dilatation.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
XS, LZ, MW: Guarantors of integrity of entire study, study concept/study design or data acquisition or data analysis/interpretation, manuscript drafting or manuscript revision for important intellectual content, manuscript final version approval; agreed to ensure any question related to the work are appropriately resolved, statistical analysis.
REFERENCES
- Budd G: On diseases of the liver. London, England: John Churchill; 1845.
- Chiari H. Ueber die selbstandige phlebitis obliterans der huaptstamme der venae hepaticae als todesurache. Beitrage Zur Pathologischen Anatomie und Zur Allgemeinen Patholgic 1899; 26:1-18.
- Ding PX, Zhang SJ, Li Z, Fu MT, Hua ZH, Zhang WG. Long-term safety and outcome of percutaneous transhepatic venous balloon angioplasty for Budd-Chiari syndrome. J Gastroenterol Hepatol 2016; 31(1):222-8. doi: 10.1111/ jgh.13025.
- Cui YF, Fu YF, Li DC, Xu H. Percutaneous recanalisation for hepatic vein-type Budd-Chiari syndrome: Long-term patency and survival. Hepatol Int 2016; 10(2):363-369. doi: 10.1007/s12072-015-9676-3.
- Valla D, Hadengue A, El Younsi M, Azar N, Zeitoun G, Boudet MJ et al. Hepatic venous outflow block caused by short-length hepatic vein stenoses. Hepatology 1997; 25(4): 814-819. doi: 10.1002/hep.510250405.
- Eapen CE, Velissaris D, Heydtmann M, Gunson B, Olliff S, Elias E. Favourable medium term outcome following hepatic vein recanalisation and/or transjugular intrahepatic portosystemic shunt for Budd Chiari syndrome. Gut 2006; 55(6):878-884. doi: 10.1136/gut.2005.071423.
- European association for the study of the liver. Electronic address eee: EASL clinical practice guidelines: Vascular diseases of the liver. J Hepatol 2016; 64:179-202.
- Wang Q, Li K, He C, Yuan X, Luo B, Qi X et al. Angioplasty with versus without routine stent placement for Budd-Chiari syndrome: A randomised controlled trial. Lancet Gastroenterology & Hepatology 2019; 4(9):686-697. doi: 10.1016/ S2468-1253(19)30177-3.
- Plessier A, Sibert A, Consigny Y, Hakime A, Zappa M, Denninger MH et al. Aiming at minimal invasiveness as a therapeutic strategy for Budd-Chiari syndrome. Hepatology 2006; 44(5):1308-1316. doi: 10.1002/hep.21354.
- Tripathi D, Macnicholas R, Kothari C, Sunderraj L, Al-Hilou H, Rangarajan B et al. Good clinical outcomes following transjugular intrahepatic portosystemic stent-shunts in Budd-Chiari syndrome. Aliment Pharmacol Ther 2014; 39(8): 864-872. doi: 10.1111/apt.12668.
- Michl P, Bilzer M, Waggershauser T, Gülberg V, Rau HG, Reiser M et al. Successful treatment of chronic Budd-Chiari syndrome with a transjugular intrahepatic portosystemic shunt. J Hepatol 2000; 32(3):516-520. doi: 10.1016/s0168- 8278(00)80405-5.
- Bachet JB, Condat B, Hagege H, Plessier A, Consigny Y, Belghiti J, et al. Long-term portosystemic shunt patency as a determinant of outcome in Budd-Chiari syndrome. J Hepatol 2007; 46(1):60-68. doi: 10.1016/j.jhep.2006.08.016.
- Zhang W, Wang QZ, Chen XW, Zhong HS, Zhang XT, Chen XD et al. Budd-Chiari syndrome in China: A 30-year retrospective study on survival from a single center. World J Gastroenterol 2018; 24(10):1134-1143. doi: 10.3748/wjg. v24.i10.1134.
- CSIR: Expert consensus on interventional diagnosis and treatment of budd chiari syndrome. Chinese J Radiol 2010; 44:345-349.
- Tripathi D, Sunderraj L, Vemala V, Mehrzad H, Zia Z, Mangat K et al. Long-term outcomes following percutaneous hepatic vein recanalisation for Budd-Chiari syndrome. Liver Int 2017; 37(1):111-120. doi: 10.1111/liv.13180.
- Zu M, Xu H, Gu Y, Zhang Q, Wei N, Xu W et al. The application and efficacy of stent place for Budd-Chiari syndrome. J Int Med 2018; 3(1):170-175. doi.org/10.19779/j.cnki.2096-3602.2018. 03.07.
- Zu M, Zhang Q, Gu Y, Li G, Xu H, Wei N et al. Revalue of the long term effect of stent in budd-chiari syndrome. Chinese J Int Radiol 2008; 206-208.
- Li DM, Yin X, Yang F, Zhang LG, Liu TG, Fu YF. Accessory hepatic vein recanalization for hepatic vein-type Budd-Chiari syndrome. Minim Invasive Ther Allied Technol 2020; 1-6. doi: 10.1080/13645706.2020.1723110.
- Chen ZK, Fan J, Cao C, Li Y. Endovascular treatment for hepatic vein-type Budd-Chiari syndrome: Effectiveness and long-term outcome. Radiol Med 2018; 123(10):799-807. doi: 10.1007/s11547-018-0907-2.
- Mukund A, Mittal K, Mondal A, Sarin SK. Anatomic recanalization of hepatic vein and inferior vena cava versus direct intrahepatic portosystemic shunt creation in budd-chiari syndrome: Overall outcome and midterm transplant-free survival. J Vasc Interv Radiol 2018; 29(6):790-9. doi: 10.1016/j.jvir.2018.01.781.
- Singh SK, Sen Sarma M, Yadav R, Kumar S, Prasad R, Yachha SK et al. Prognostic scoring systems and outcome of endovascular radiological intervention of chronic Budd-Chiari syndrome in children. Liver Int 2018; 38(7):1308-15. doi: 10.1111/liv.13683.
- Fan X, Liu K, Che Y, Wang S, Wu X, Cao J et al. Good clinical outcomes in budd-chiari syndrome with hepatic vein occlusion. Dig Dis Sci 2016; 61(10):3054-060. doi: 10.1007 /s10620-016-4208-0.
- Sang HF, Li XQ. Endovascular treatment of Budd-Chiari syndrome with hepatic vein obstruction in China. J Laparoendosc Adv Surg Tech A 2014; 24(12):846-851. doi: 10.1089/ lap.2014.0095.
- Kathuria R, Srivastava A, Yachha SK, Poddar U, Baijal SS. Budd-Chiari syndrome in children: clinical features, percutaneous radiological intervention, and outcome. Eur J Gastroenterol Hepatol 2014; 26(9):1030-38. doi: 10.1097/MEG. 0000000000000144.
- Zhang B, Jiang ZB, Huang MS, Zhu KS, Qian JS, Shan H. Effects of percutaneous transhepatic interventional treatment for symptomatic Budd-Chiari syndrome secondary to hepatic venous obstruction. J Vasc Surg Venous Lymphat Disord 2013; 1(4):392-399. doi: 10.1016/j.jvsv.2013.05.008.
- Eldorry A, Barakat E, Abdella H, Abdelhakam S, Shaker M, Hamed A et al. Outcome of non surgical hepatic decompression procedures in Egyptian patients with Budd-Chiari. World J Gastroenterol 2011; 17(7):906-913. doi: 10.3748/wjg. v17.i7.906.
- Li T, Zhai S, Pang Z, Ma X, Cao H, Bai W et al. Feasibility and midterm outcomes of percutaneous transhepatic balloon angioplasty for symptomatic Budd-Chiari syndrome secondary to hepatic venous obstruction. J Vasc Surg 2009; 50(5):1079-1084. doi: 10.1016/j.jvs.2009.06.049.
- Yu C, Gao Y, Zhou W, Nie Z, Yu L. Treatment of 16 cases of Budd Chiari syndrome of hepatic vein type by transjugular and transhepatic approach. J Practical Med 2008; 24: 3203-3204.
- Zhengran L, Hong S, Kangshun Z. Interventional treatment for buddi-chiari syndrome with occlusive hepatic veins. J Int Radiol 2004; 13:25-27.