Response to Trastuzumab Treatment and Number of Cycles ın Her2-Positive Metastatic Gastric Cancer Survival
By Fatma Bugdayci Basal1, Cengiz Karacin1, Irem Bilgetekin1, Omur Berna Oksuzoglu1, Umut Demirci2Affiliations
doi: 10.29271/jcpsp.2020.12.1279ABSTRACT
Objective: To evaluate the effects of trastuzumab on overall survival (OS) and progression-free survival (PFS) in patients with HER2-positive metastatic gastric cancer.
Study Design: Descriptive study.
Place and Duration of Study: Department of Medical Oncology, Dr. Abdurrahman Yurtaslan Oncology Training and Research Hospital, from January 2012 to December 2019.
Methodology: Medical records of 33 HER2-positive metastatic gastric cancer patients who had received trastuzumab combined with chemotherapy at least 6 cycles and/or followed by maintenance as the first-line treatment were examined. Kaplan–Meier method was used for survival analysis. Log-Rank test was used to compare survival times. The prognostic factors were determined by Cox regression analysis.
Results: The median OS was 15.97 months, and the median PFS was 11.11 months. The median OS and PFS were significantly higher in those who demonstrated partial or complete response to trastuzumab combination treatment, and those who received more than 10 cycles of trastuzumab. A Cox regression analysis revealed that the risk of death was 3.18 times higher in patients with stable disease and 0.44 times lower in patients who received more than 10 cycles of trastuzumab as maintenance.
Conclusion: The OS time was prolonged when the partial and complete response was obtained with chemotherapy combined with trastuzumab, as well as OS and PFS times were improved with the increasing number of trastuzumab cycles. Thus, it is concluded that the response better than stable disease of trastuzumab treatment and an increased number of cycles predicts improved survival efficiently in patients with HER2-positive metastatic gastric cancer.
Key Words: Metastatic gastric cancer, Trastuzumab, Prognosis, Response, Efficiency.
INTRODUCTION
Gastric cancer is the fifth most commonly diagnosed cancer in the world and the third leading cause of cancer deaths.1,2 Unfortunately, there is no globally standardised regimen for treating advanced stage gastric cancer.3
Advanced gastric cancer patients often experience insufficient response to treatment, rapid progression of the disease, and toxicities related to treatment. Therefore, it is of utmost importance to identify potential therapeutic goals and targeted therapies for improving the outcomes of systemic treatment beyond the results achieved by traditional chemotherapy.4,5
The primary function of HER2 is to mediate cell growth, differentiation, and survival. Therefore, HER2-positive tumors are expected to have more aggressive features compared to those that are HER2-negative.6 The frequency of HER2 overexpression in gastric cancer is between 6% and 30%.4 Trastuzumab is a monoclonal antibody that specifically targets the HER2 protein by directly binding to the extracellular part of the receptor which leads to inhibition of angiogenesis, and induction of apoptosis.7,8
The TOGA study was the first large scale, randomised, and controlled phase III study that evaluated the addition of trastuzumab to platin-based chemotherapy in HER2-positive advanced stage gastric cancer patients and indicates that trastuzumab treatment in combination with chemotherapy may be a new option for patients with HER2-positive advanced stage gastric or gastroesophageal junction cancers.9
The aim of the current study was to evaluate the effects of trastuzumab treatment in combination with chemotherapy and/or followed by maintenance therapy on OS and PFS in patients with HER2-positive metastatic gastric cancer in a real-life cohort at a single centre.
METHODOLOGY
In this retrospective study, 38 patients diagnosed with HER2-positive metastatic gastric cancer in the Medical Oncology Clinic of Dr. Abdurrahman Yurtaslan Oncology Training and Research Hospital, between January 2012 and December 2019, were analysed. Based on inclusion criteria, a total of 33 patients were included in this study. Inclusion criteria were as follows: having a diagnosis of metastatic gastric cancer, detection of HER2 by fluorescence in situ hybridisation (FISH) or silver-enhanced in situ hybridisation (SISH), and having received 6 cycles of trastuzumab with chemotherapy followed by maintenance or not as the first-line therapy for metastatic stage. Patients were excluded from the study, if they had gastric cancer with mixed histology, had neuroendocrine or mixed adeno-neuroendocrine carcinoma (MANEC), had received less than 6 cycles of combination therapy, had progressive disease during combination therapy, were HER2 positive based on immunohistochemistry (IHC) but negative for HER2 via FISH or SISH, or if they had missing medical records. The data of 33 patients meeting inclusion criteria were analysed. Demographic and clinicopathologic data were recorded from the patients’ records and information system at the hospital.
Treatment response was assessed according to RECIST version 1.1 (Response Evaluation Criteria in Solid Tumors) criteria,10 and was classified as either complete response, partial response, and stable disease. The response to treatment was categorised as either complete/partial response or stable disease.
The primary end point OS was defined as the period from the initiation of treatment to death, or to the last follow-up date for surviving patients. The primary endpoint PFS was defined as the period from the initiation of treatment to progression date, or to the last follow-up date for non-progressive patients.
All analyses were conducted using SPSS version 21 (SPSS Inc., Chicago, IL, USA). Qualitative data were expressed as frequency and percentage while quantitative as mean ± S.D and median (IQR). Survival analysis was performed using the Kaplan-Meier method. Log-Rank test was used to compare survival times between groups. The prognostic factors important for death and progression were determined by Cox regression analysis. Values of p <0.05 were accepted as statistically significant for all analyses.
RESULTS
All the general demographic and clinical characteristics of the included patients are presented in Table I.
Over a median follow-up period of 14.6 months (IQR: 8.2 – 20.4), the median OS was 15.97 months (95% CI: 9.54- 22.39 months) and the median PFS was 11.11 months (95% CI: 6.85- 15.36).
Univariate analyses did not reveal any significant correlation for age, gender, family history, performance score at diagnosis, tumor localisation, stage, subtype, pathological type, grade, adjuvant treatment status, and development of carcinomatosis peritonei and the presence of liver metastasis for both OS and PFS.
Table I: General demographic and clinical characteristics of patients.
|
Age, year* |
62.36 ± 10.64 |
|
|
Gender |
||
|
Male |
27 (81.82%) |
|
|
Female |
6 (18.18%) |
|
|
Family history, yes |
8 (24.24%) |
|
|
Performance score at the time of diagnosis |
||
|
0 |
3 (9.09%) |
|
|
1 |
27 (81.82%) |
|
|
2 |
3 (9.09%) |
|
|
Localisation |
||
|
Cardia |
17 (51.52%) |
|
|
Corpus |
9 (27.27%) |
|
|
Antrum |
7 (21.21%) |
|
|
Stage at diagnosis |
||
|
Stage III |
4 (12.12%) |
|
|
Stage IV |
29 (87.88%) |
|
|
Biopsy type |
||
|
Endoscopic |
29 (87.88%) |
|
|
Surgical |
4 (12.12%) |
|
|
Pathological type |
||
|
Adeno Ca |
30 (90.91%) |
|
|
Signet ring cell ca |
3 (9.09%) |
|
|
Sub-Type |
||
|
Diffuse |
5 (15.15%) |
|
|
Intestinal |
28 (84.85%) |
|
|
Grade |
||
|
II |
3 (9.09%) |
|
|
III |
30 (90.91%) |
|
|
Surgery |
||
|
None |
24 (72.73%) |
|
|
Subtotal gastrectomy |
4 (12.12%) |
|
|
Total gastrectomy |
5 (15.15%) |
|
|
Number of dissected lymph nodes |
24 (IQR:22.5 - 26) |
|
|
Number of metastatic lymph nodes |
12 (IQR:8 - 17.5) |
|
|
Serosa ınvasion |
9 (27.27%) |
|
|
Lymphovascular ınvasion |
9 (27.27%) |
|
|
Perineural ınvasion |
9 (27.27%) |
|
|
Surgical border |
||
|
Negative |
7 (77.78%) |
|
|
Positive |
2 (22.22%) |
|
|
Adjuvant therapy |
3 (9.09%) |
|
|
Metastasis |
33 (100.00%) |
|
|
Liver |
27 (81.82%) |
|
|
Distant lymph node |
13 (39.39%) |
|
|
Lung |
7 (21.21%) |
|
|
Bone |
4 (12.12%) |
|
|
Number of metastatic sites |
||
|
|
<2 |
17 (51.52%) |
|
|
≥2 |
16 (48.48%) |
|
Carcinomatosis peritonei |
5 (15.15%) |
|
|
Number of trastuzumab cycles |
10 (IQR:6 – 16) |
|
|
Response to trastuzumab treatment |
||
|
CR |
1 (3.03%) |
|
|
PR |
16 (48.48%) |
|
|
SD |
16 (48.48%) |
|
| Continued... | ||
|
Chemotherapy regimes |
||
|
Cisplatin+5FU based/ herceptin |
24(72.73%) |
|
|
|
Oxaliplatin+5FU based/ herceptin |
5 (15.15%) |
|
Carboplatin+5FU based/ herceptin |
4 (12.12%) |
|
|
Progression |
32 (97%) |
|
|
Endstate |
||
|
Alive |
5 (15.15%) |
|
|
Exitus |
28 (84.85%) |
|
|
*Age was given as mean ± standard deviation due to normal distribution, CR: Complete Response, PR: Partial Response, SD: Stable Diseas |
||
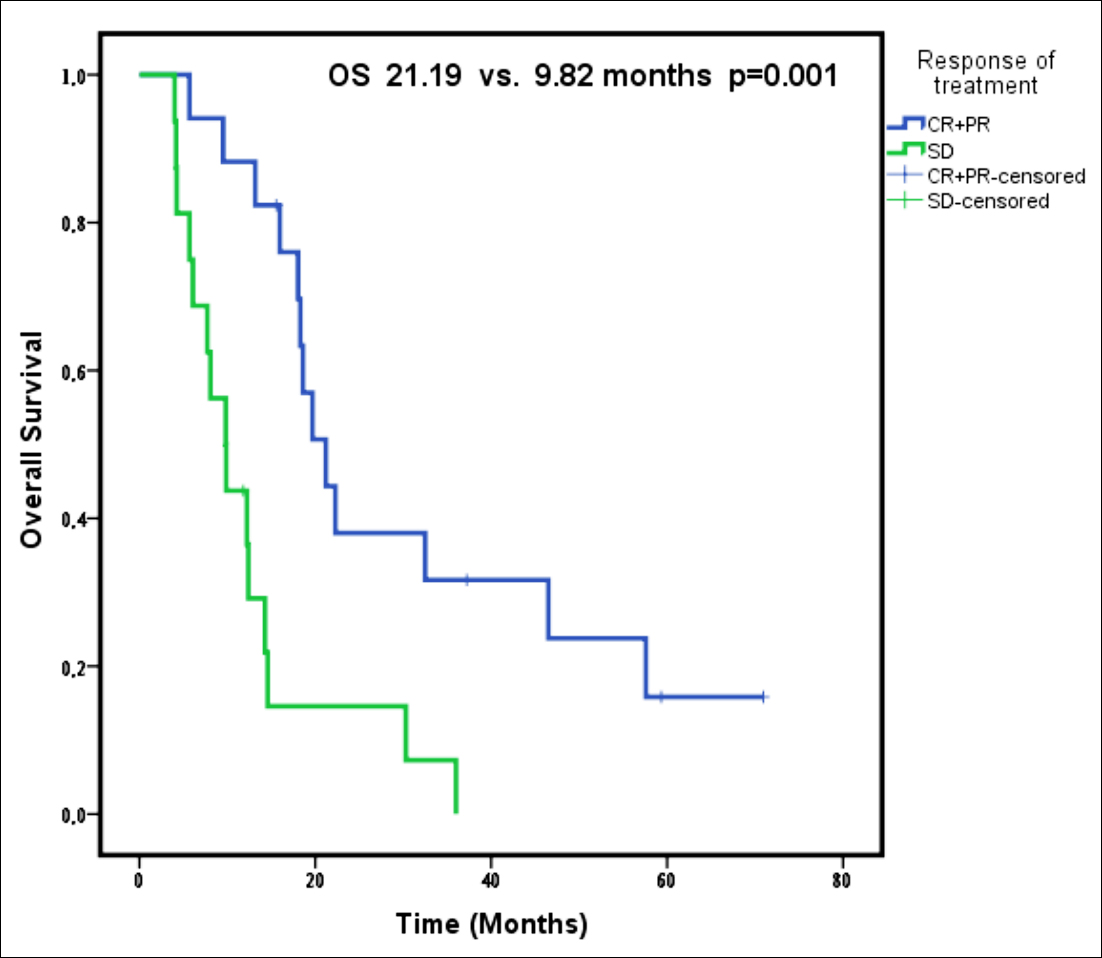 Figure 1: Overall survival according to response to the treatment.
Figure 1: Overall survival according to response to the treatment.
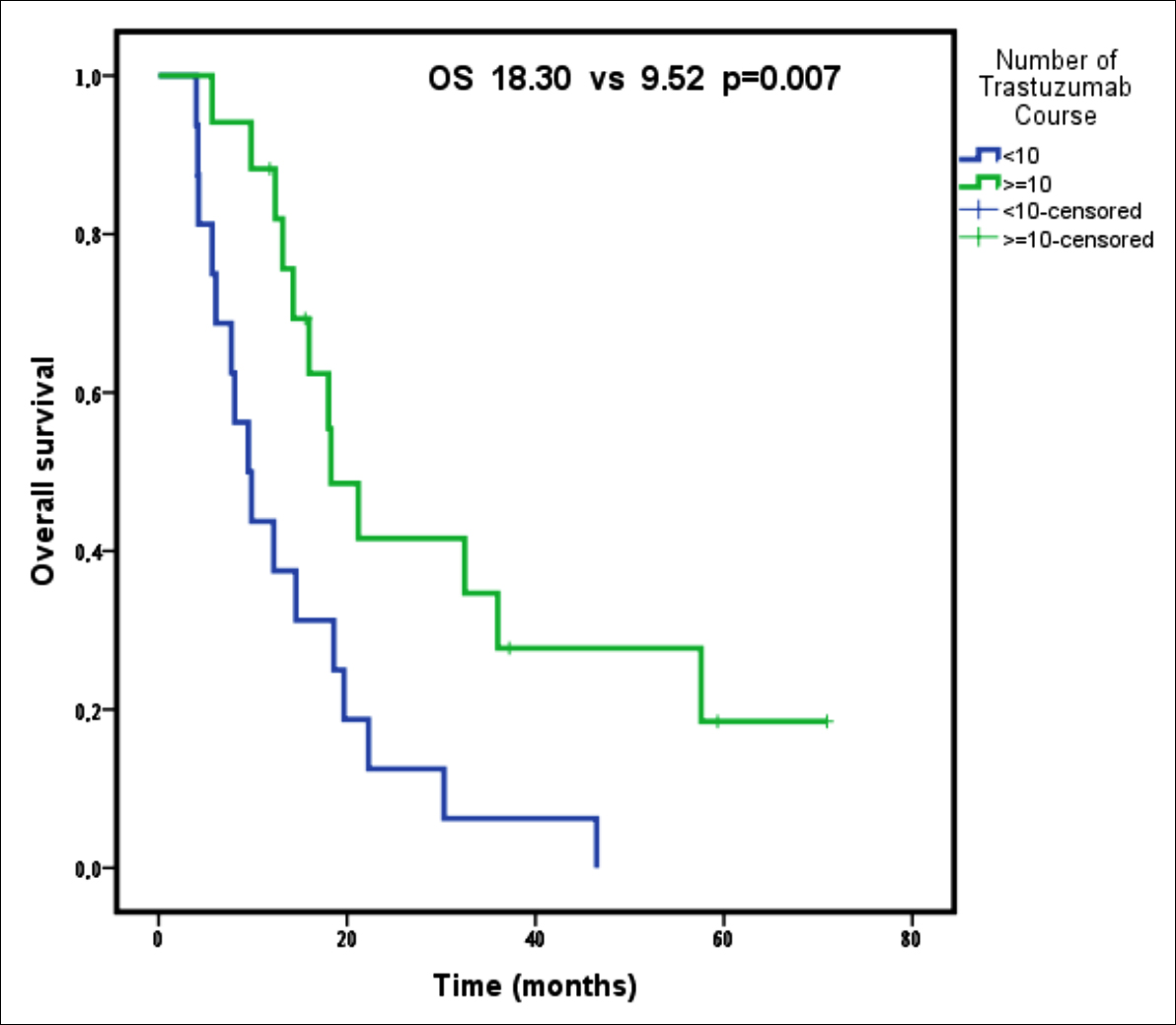 Figure 2: Overall survival according to the number of trastuzumab courses.
Figure 2: Overall survival according to the number of trastuzumab courses.
However, the median OS was significantly longer in patients with partial and complete response to trastuzumab treatment compared to those with stable disease (p = 0.001, Table II, Figure 1). In addition, presence of received trastuzumab courses more than 10 cycles provided an OS advantage (p = 0.007, Table II, Figure 2). Furthermore, the patients who had single organ metastasis survived longer significantly (p = 0.031, Table II). The PFS was significantly longer in patients undergoing surgical treatment (p= 0.010, Table II), and those who showed complete and/or partial response to trastuzumab treatment (p = 0.007, Table II), and those who received more than 10 courses of trastuzumab treatment (p = 0.001, Table II).
A Cox regression analysis revealed that patients who had received trastuzumab treatment more than 10 courses had decreased risk of death (0.442 times lower, p = 0.047). Furthermore, mortality risk increased in patients without PR or CR (3.184 times higher, p = 0.006).
Based on the Cox regression analysis performed using progression-free survival time, the progression risk decreased with the increasing number of trastuzumab cycles (0.364 times lower for more than 10 courses of trastuzumab, p = 0.021), and in addition, the progression risk increased in elderly patients (2.355 times higher for patients who >65 years old, p = 0.039).
DISCUSSION
Gastric cancer is a heterogeneous disease caused by genomic changes that can induce the activation of a large number of molecular pathways. There have been significant advances in the therapeutic approach to gastric cancer due to the identification of a subgroup with overexpressed and amplified HER2.11 The TOGA study was the first randomised study to confirm that trastuzumab could be successfully used to treat HER2-positive gastric cancer.9 In the present study, the authors investigated the survival outcomes of trastuzumab treatment in combination with chemotherapy and/or followed by maintenance in patients with HER2-positive metastatic gastric cancer.
In the current study, the OS and PFS were longer than those reported in the TOGA study.9 When compared to the TOGA study, the performance status of patients receiving trastuzumab treatment in the current study is similar. However, 77% of patients in the TOGA study had intestinal histological type gastric cancer, while in our current study this ratio was 84.85%. HER2 expression is more common in intestinal type tumors, and it has been reported that it has better treatment results than diffuse type tumors.9,12 This could be one of the reasons that the current study had better survival results as compared to the TOGA study. Furthermore, the median number of trastuzumab cycles in the TOGA study (8 cycles (range 1-49)) was lower than that of the current study group (10 cycles (range 6-38)). In addition, the ratio of patients with a good response to treatment was higher in the current study group (51.52%) compared to the TOGA study (47%), which may be one of the reasons that explain the longer survival periods found in the present study. Furthermore, evaluating only the patients who those received maintenance therapy might improved the results of our study.
Table II: Results of the univariate analyses of prognostic factors for PFS and OS.
|
|
PFS, months median (95% CI) |
p |
OS, months median (95% CI) |
p |
|
|
All patients |
11.10 (6.85-15.35) |
N/A |
15.96 (9.54-22.39) |
N/A |
|
|
Age group |
|||||
|
≤ 65 |
14.25 (10.37-18.14) |
0.066 |
18.30 (8.05-28.54) |
0.285 |
|
|
> 65 |
8.34 (5.14-11.54) |
12.38 (8.36-16.40) |
|||
|
Gender |
|||||
|
Male |
11.89 (5.48-18.30) |
0.964 |
14.29 (10.56-18.03) |
0.375 |
|
|
Female |
10.94 (6.68-15.19) |
22.28 (14.60-29.95) |
|||
|
Family story |
|||||
|
No |
11.89 (8.67-15.11) |
0.457 |
18.03 (11.75-24.31) |
0.537 |
|
|
Yes |
8.34 (4.56-12.12) |
12.22 (5.66-18.77) |
|||
|
Performance * |
|||||
|
≤1 |
11.10 (8.45-13.75) |
0.910 |
15.96 (9.75-22.17) |
0.716 |
|
|
>1 |
6.07 (3.02-9.12) |
6.07 (3.02-9.12) |
|||
|
Localisation |
|||||
|
Cardia |
10.31 (6.65-13.98) |
0.506 |
12.38 (7.92-16.84) |
0.206 |
|
|
Corpus |
12.81 (0.23-25.38) |
19.68 (0.00-58.91) |
|||
|
Antrum |
16.23 (5.10-27.35) |
18.03 (12.72-23.34) |
|||
|
Stage at diagnosis |
|||||
|
Stage III |
14.58 (0.00-37.19) |
0.437 |
19.68 (0.00-40.67) |
0.294 |
|
|
Stage IV |
10.94 (6.37-15.50) |
14.29 (9.66-18.91) |
|||
|
Pathological type |
|||||
|
Adeno Ca |
10.94 (6.39-15.48) |
0.935 |
15.96 (9.65-22.28) |
0.616 |
|
|
Signet ring cell Ca |
14.58 (3.59-25.57) |
14.58 (3.59-25.57) |
|||
|
Sub-type |
|||||
|
Diffuse |
14.58 (10.77-18.39) |
0.583 |
14.58 (13.95-15.22) |
0.792 |
|
|
Intestinal |
10.31 (6.86-13.76) |
15.96 (8.63-23.30) |
|||
|
Grade |
|||||
|
II |
14.25 (0.00-30.66) |
0.710 |
18.03 (0.00-40.48) |
0.446 |
|
|
III |
10.94 (6.39-15.48) |
14.58 (8.21-20.96) |
|||
|
Surgery |
|||||
|
No |
8.34 (5.23-11.46) |
0.010 |
12.22 (8.41-16.03) |
0.060 |
|
|
Yes |
17.24 (9.47-25.02) |
19.68 (14.88-24.47) |
|||
|
Adjuvant chemotherapy |
|||||
|
No |
10.31 (6.74-13.88) |
0.287 |
14.29 (7.29-21.29) |
0.260 |
|
|
Yes |
31.50 (4.43-58.58) |
36.00 (1.72-70.28) |
|
||
|
Liver metastasis |
|||||
|
|
No |
7.59 (0.00-15.67) |
0.491 |
8.08 (0.00-17.9) |
0.320 |
|
|
Yes |
11.10 (8.43-13.78) |
18.03 (11.76-24.31) |
||
|
Number of metastatic sites |
|||||
|
|
<2 |
14.26 (5.45-23.07) |
0.051 |
18.30 (9.14-27.46) |
0.031 |
|
|
≥2 |
8.44 (3.36-13.53) |
13.17 (9.12-17.23) |
||
|
Carcinomatosis peritonei |
|||||
|
No |
11.89 (9.46-14.32) |
0.329 |
18.03 (12.19-23.87) |
0.152 |
|
|
Yes |
7.58 (4.34-10.83) |
8.08 (3.77-12.38) |
|||
|
Response to trastuzumab treatment |
|||||
|
Complete & partial response |
16.23 (10.07-22.38) |
0.007 |
21.19 (16.20-26.18) |
0.001 |
|
|
Stable disease |
7.72 (6.49-8.94) |
9.82 (6.34-13.30) |
|||
|
Median number of trastuzumab courses |
|||||
|
|
<10 |
7.72 (6.49-8.94) |
0.001 |
9.52 (6.05-13.00) |
0.007 |
|
|
≥10 |
16.23 (10.65-21.80) |
18.30 (12.56-24.03) |
||
|
PFS: Progression-free survival. OS: Overall survival; CI: Confidence interval. N/A: Not applicable. Ca: Carcinoma. |
|||||
In the univariate analyses, no assosiciation was found between patient characteristics and survivals. Similarly, Gong et al. reported no correlation between OS and age, ECOG performance score, primary disease localization, disease stage, liver metastasis, and previous gastrectomy; however, that study did report a positive correlation between OS and HER2/CEP17 ratio.13 The detection of HER2 by FISH or SISH was one of the inclusion criteria in this study.
In another study, it was indicated that receiving trastuzumab, fluoropyrimidine, taxanes, and irinotecan therapy had a positive effect on OS.6 In the present study, types of combination chemotherapy did not provide a significant difference in OS and PFS. In a retrospective study including 33 patients conducted by Mutlu et al., Cox regression analysis revealed that a family history of cancer, tumor localisation, histological type, number of metastases, performance status, and previous resection of the tumor had no effect on OS and PFS, whereas the number of trastuzumab cycles had a positive effect on OS and PFS similar to the current study.14 Different studies have reported that various factors affect survival.14,15 This may be due to varying patient characteristics throughout these studies, and different variables included in multivariate analysis models. Results of our study showed that the survival is prolonged in HER2-positive advanced gastric cancer patients with a better response to trastuzumab treatment and a higher number of trastuzumab cycles.
Trastuzumab has led to significant progress in the treatment of gastric cancer with HER2 overexpression, and the TOGA study has been one of the most important studies in this regard. 11 Three Phase II studies and one retrospective study evaluating the results of trastuzumab therapy with various chemotherapy regiments in HER2-positive advanced stage gastric cancer have reported results similar to those of the TOGA study.13,16,17 The combination of chemotherapy regimens were cisplatin, oxaliplatin and carboplatin based in the present study.
The key limitations of this study are its retrospective study design, small number of cases examined, patients from a single centre, and the absence of a control group. However, multivariate analyses were used to overcome these limitations. In addition, although this was a single-centre retrospective study with a relatively small number of cases, this research is valuable as it demonstrates effective treatment results for trastuzumab in HER2-positive gastric cancer patients over a long period of time.
CONCLUSION
In the present study, partial and/or complete response to trastuzumab treatment and a higher number of trastuzumab cycles prolonged OS. Moreover, PFS was prolonged as the number of trastuzumab cycles increased. Trastuzumab treatment is beneficial and can be used in the first-line treatment of HER2-positive metastatic gastric cancers.
ETHICAL APPROVAL:
Ethics Committee approval was received from the Ethics Committee of University of Health Sciences, Dr Abdurrahman Yurtaslan Oncology Training and Research Hospital
(No. E-75, July 30, 2019).
PATIENTS’ CONSENT:
Informed consents were obtained from all participants or their family included in the study.
CONFLICT OF INTEREST:
Authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
FBB: Conception and design, analyses and interpretation of data, drafting of manuscript.
CK: Analysis and interpretation of data.
IB: Acquisition of data.
OBO: Advices and final approval.
UD: Conception and design, reviewed the paper, and final approval.
REFERENCES
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA and Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6):394-424. doi: 10.3322/caac.21492.
- Tulay E, Karacin C, Gokhan U, Ergun Y, Yazici O, Imamoglu GI, et al. Efficacy of the combination of modified docetaxel, cisplatin and fluorouracil in locally advanced gastric cancer: Evaluation of real-life outcomes. UHOD 2020; 29: 001-9.
- Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer 2014; 110(5): 1163-8. doi: 10.1038/bjc.2014.18.
- Kelly CM and Janjigian YY. The genomics and therapeutics of HER2-positive gastric cancer—from trastuzumab and beyond. J Gastrointest Oncol 2016; 7(5):750. doi: 10.21037/jgo.2016.06.10.
- Chua TC and Merrett ND. Clinicopathologic factors associated with HER2‐positive gastric cancer and its impact on survival outcomes - A systematic review. Int J Cancer 2012; 130(12):2845-56. doi: 10.1002/ijc.26292.
- Shitara K, Yatabe Y, Matsuo K, Sugano M, Kondo C, Takahari D, et al. Prognosis of patients with advanced gastric cancer by HER2 status and trastuzumab treatment. Gastric Cancer 2013; 16(2):261-7. doi: 10.1007/s10120-012-0179-9.
- Gravalos C and Jimeno A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann Oncol 2008; 19(9):1523-9. doi: 10.1093/annonc/mdn169.
- Pazo Cid RA and Antón A. Advanced HER2-positive gastric cancer: Current and future targeted therapies. Crit Rev Oncol Hematol 2013; 85(3):350-62. doi: 10.1016/j.critrevonc.2012.08.008.
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): Aphase 3, open-label, randomised controlled trial. Lancet 2010; 376(9742):687-97. doi: 10.1016/S0140-6736(10)61121-X.
- Therasse P, Eisenhauer EA and Verweij J. RECIST revisited: A review of validation studies on tumour assessment. Eur J Cancer 2006; 42(8):1031-9. doi: 10.1016/j.ejca.2006.01. 026.
- Palle J, Rochand A, Pernot S, Gallois C, Taïeb J and Zaanan A. Human epidermal growth factor receptor 2 (HER2) in advanced gastric cancer: Current knowledge and future perspectives. Drugs 2020; 80(4):1-15. doi: 10.1007/ s40265-020-01272-5.
- Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, et al. HER2 screening data from ToGA: Targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 2015; 18(3):476-84. doi: 10.1007/s10120-014-0402-y.
- Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/ capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): A multicenter, phase II trial. BMC Cancer 2016; 16:68. doi: 10.1186/s12885- 016-2092-9.
- Ilhan-Mutlu A, Taghizadeh H, Beer A, Dolak W, Ba-Ssalamah A, Schoppmann SF, et al. Correlation of trastuzumab-based treatment with clinical characteristics and prognosis in HER2-positive gastric and gastroesophageal junction cancer: A retrospective single center analysis. Cancer Biol Ther 2018; 19(3):169-74. doi: 10.1080/15384047.2017. 1414759.
- Yi JH, Kang JH, Hwang IG, Ahn HK, Baek HJ, Lee SI, et al. A retrospective analysis for patients with her2-positive gastric cancer who were treated with trastuzumab-based chemotherapy: In the perspectives of ethnicity and histology. Cancer Res Treat 2016; 48(2):553-60. doi: 10.4143/crt.2015.155.
- Soularue E, Cohen R, Tournigand C, Zaanan A, Louvet C, Bachet JB, et al. Efficacy and safety of trastuzumab in combination with oxaliplatin and fluorouracil-based chemotherapy for patients with HER2-positive metastatic gastric and gastro-oesophageal junction adenocarcinoma patients: A retrospective study. Bull Cancer 2015; 102(4):324-31. doi: 10.1016/j.bulcan.2014.08.001.
- Ryu MH, Yoo C, Kim JG, Ryoo BY, Park YS, Park SR, et al. Multicenter phase II study of trastuzumab in combination with capecitabine and oxaliplatin for advanced gastric cancer. Eur J Cancer 2015; 51:482-8.