Uterine Adenosarcoma in a Teen Ager
By Yuanyuan Wang1, Yongliang Teng2, Jie Li1, Ye Yuan1Affiliations
doi: 10.29271/jcpsp.2022.04.18ABSTRACT
Uterine adenosarcoma is a rare tumor composed of benign epithelium in combination with malignant mesenchymal components. Misdiagnosis is common due to the lack of specific clinical manifestations and the rarity of such tumors in young age groups. The mean age at diagnosis is 58.6 years, and <10% of patients are younger than 40 years. Herein, we describe a case of a 19-year girl who presented with worsening menorrhagia. Her ultrasound revealed multiple heterogeneous masses in the uterine cavity. Intraoperatively, a small piece of grayish-white tumor tissue was sent for the frozen section, but the results did not confirm malignancy. She underwent hysteroscopy, laparoscopic hysterectomy, and bilateral salpingo-oophorectomy. Histopathologic examination finally confirmed uterine adenosarcoma. The patient had no evidence of residual tumor or recurrence during six months of follow-up.
Key Words: Uterus, Adenosarcoma, Mixed tumor.
INTRODUCTION
Uterine sarcomas generally account for approximately 1% of all gynecologic malignancies. Among these, the subtype adenosarcoma comprises only 8% of all uterine sarcomas. Clement and Scully initially reported this tumor as Mullerian adenosarcoma in 1974,1 which is characterised by having both benign epithelial and low-grade malignant stromal components. Uterine adenosarcoma usually occurs in perimenopausal or postmenopausal women, and less than 10% of patients are younger than 40 years. Due to its rarity and nonspecificity of clinical characteristics and tumor markers, misdiagnosis of the tumor is common. Herein, we present the minimally invasive treatment of a uterine adenosarcoma by hysteroscopy and laparoscopy in a 19- year girl.
CASE REPORT
A 19-year girl, otherwise fit and well, presented with progressively worsening menorrhagia for three months. She had no history of hormonal treatment or any other major illness. There was no family history of cancer or other diseases. Physical examination did not reveal any significant abdominal tenderness, palpable mass or other findings.
A transabdominal ultrasound scan revealed multiple heterogeneous masses occupying the uterine cavity. Hysteroscopy was performed, which revealed multiple polypoid masses in the uterine cavity with the largest measuring 1×0.5×0.5 cm. A small piece of grayish-white tumor tissue was sent for the frozen section, which suggested a stromal tumor with malignant potential. Hysterectomy and bilateral salpingo-oophorectomy were performed. No lymphadenectomy was performed. Histopathology showed leaf-like or club-like stromal projections lined by epithelium, the architecture resembling that of a phyllodes tumor of the breast. The stroma was hypercellular with up to 1–6 mitoses/10 high power fields (HPFs). There were focal areas with superficial myometrial invasion, but no evidence of regional lymphadenopathies or infiltration of surrounding tissues. An immunohistochemical examination showed positive staining for CD10, ER, PR, and negative staining for H-caldesmon, Desmin, Cyclin D1, pan-CK, SMA, and CK-AE1/AE3. The Ki-67 proliferation index was approximately 5% in the hot spots. The final diagnosis was confirmed as a primary uterine adenosarcoma (Figures 1-2). The postoperative PET-CT did not detect any distant metastases. The patient received no further treatment after surgery with no evidence of residual tumor or recurrence during the 6-month follow-up.
All clinical investigations were conducted in accordance with the principles expressed in the Declaration of Helsinki.
DISCUSSION
Adenosarcomas of the uterus are usually present in perimenopausal or postmenopausal women with 58.6 years being the reported mean age of the presentation.2 The main complaint is irregular vaginal bleeding occurring in approximately 65 to 76% of cases and ranging from spotting to menorrhagia. However, some patients present with other nonspecific symptoms, such as pelvic pain, vaginal discharge, and symptoms related to uterine enlargement. A high percentage of cases is asymptomatic.
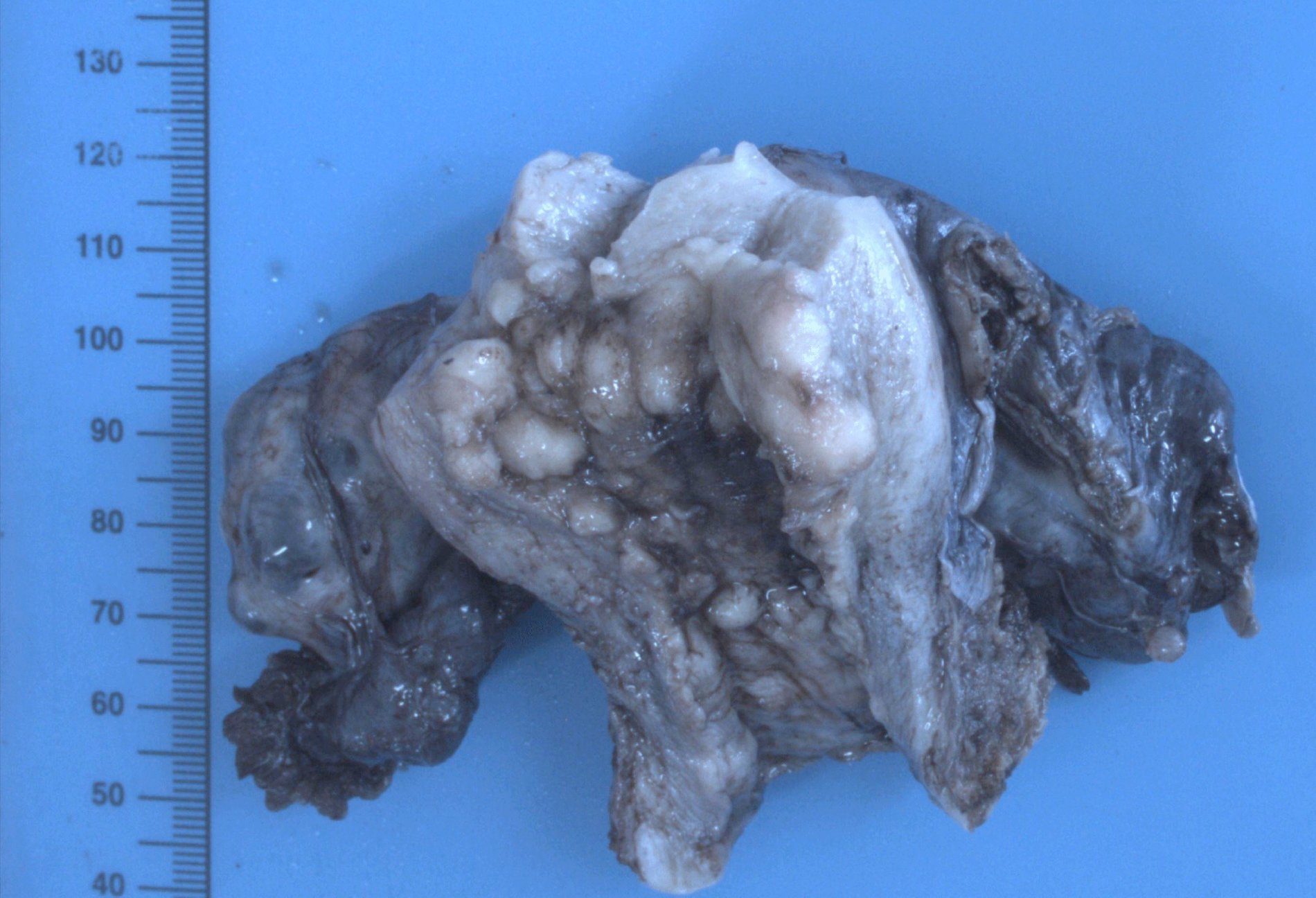 Figure 1: Macroscopic features of the tumor showing a multi-nodular grayish-white mass.
Figure 1: Macroscopic features of the tumor showing a multi-nodular grayish-white mass.
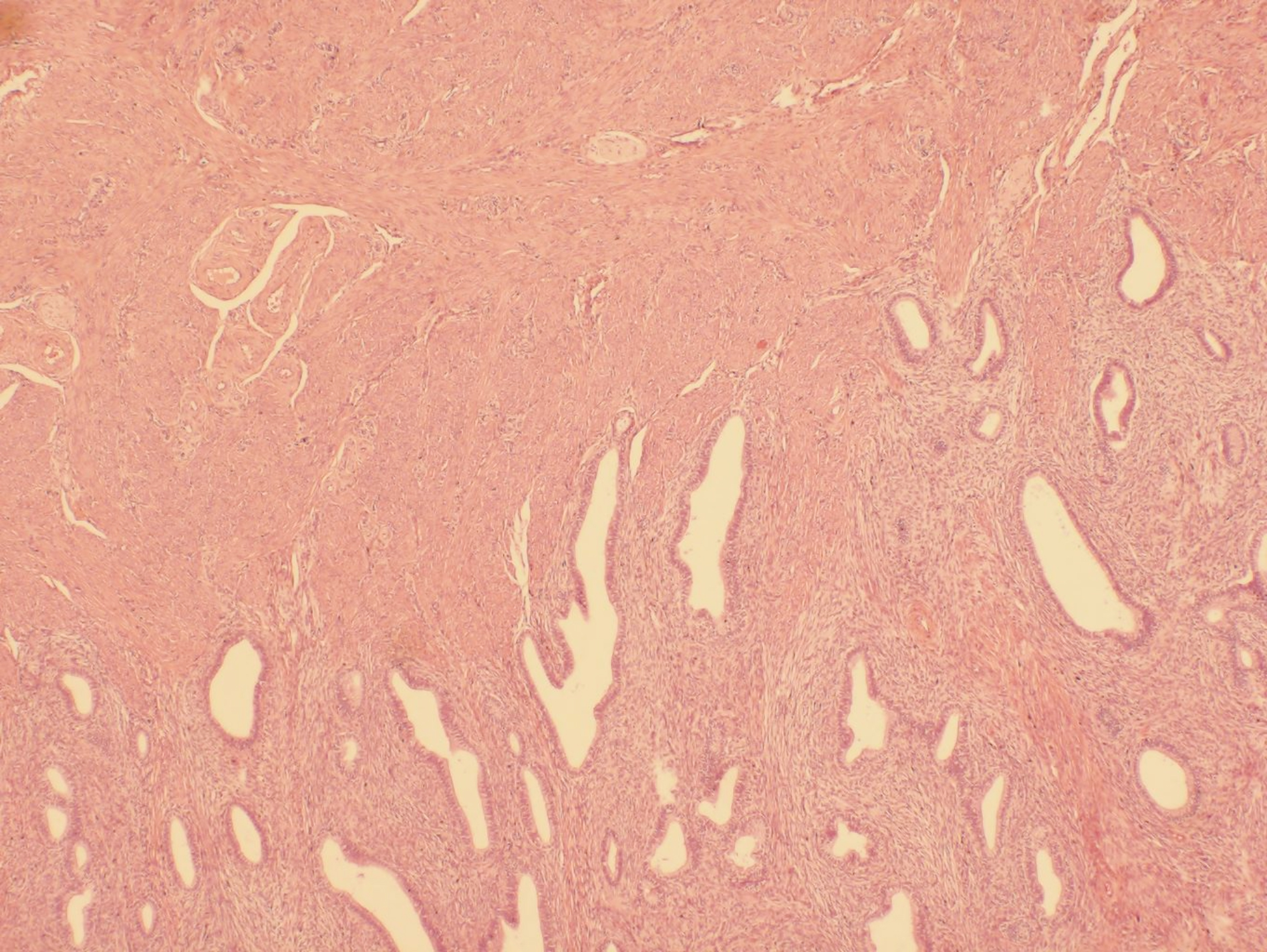 Figure 2: A biphasic tumor composed of benign epithelial elements and a sarcomatous stroma (H&E, ×40).
Figure 2: A biphasic tumor composed of benign epithelial elements and a sarcomatous stroma (H&E, ×40).
The confident diagnosis of uterine adenosarcoma is completely dependent on the characteristic morphological appearance.3 Adenosarcoma is exemplified by the presence of both a malignant mesenchymal component and an epithelial element that is benign but neoplastic. Histologically, the architecture often demonstrates leaf-like or club-like papillary projections, formed by the stroma compressing the epithelium. The epithelial elements of papillary projections are surrounded by abundant stromal or mesenchymal components, forming peri-glandular cuffs or so-called “cambium layer”, which is one of the most useful clues suggesting a diagnosis of adenosarcoma.4 A stromal mitotic rate of ≥ 2 per 10 HPFs is usually required by the World Health Organization (WHO) criteria to make the diagnosis of adenosarcoma. But in practice, if the characteristic leaf-like architecture and cambium layer are present with periglandular cuffing, the diagnosis of adenosarcoma is possible. 5 Adenosarcomas with more than 25% of the pure sarcoma component (without epithelial elements) are termed adenosarcomas with sarcomatous overgrowth. 6
Due to the rarity of adenosarcomas, there is no consensus regarding therapy. The current standard for management strategy of uterine adenosarcomas is surgical resection, comprising of hysterectomy and bilateral salpingo-oophorectomy in the early stage with five-year survival approaching 50%.7 Whether or not to keep ovaries is still under debate. There is no role of adjuvant treatment in the early stage but chemotherapy has a role in recurrent or metastatic disease. Most cases are cured, but sarcomatous overgrowth, myometrial infiltration, adjacent organ invasion and distant metastases are proposed to be predictors of an unfavourable outcome. Unfortunately, local recurrence is common, and is associated with poor prognosis, particularly in those cases with sarcomatous overgrowth (45-70% versus 15-25% for those without sarcomatous overgrowth).
In conclusion, uterine adenosarcoma is rare and difficult to distinguish from other uterine neoplasms. Gynecologists and pathologists should be aware of the existence of this uncommon tumor and take any suspicious details into consideration to obtain a correct diagnosis. Currently, total abdominal hysterectomy with bilateral salpingo-oophorectomy remains the mainstay of the treatment.
ETHICAL APPROVAL:
This study was approved by the Ethics Committee of the First Hospital of Jilin University (Changchun, People’s Republic of China), and was permitted to be published.
PATIENT’S CONSENT:
Written informed consent to have the case details and accompanying images published was obtained from the patient.
CONFLICT OF INTEREST:
The author declared no conflicts of interest.
AUTHORS’ CONTRIBUTION:
YW, YY: Designed the report.
YW, JL, YT: Collected the patient’s clinical data and information.
YW, YY, YT: Wrote the paper.
All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
REFERENCES
- Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-99. Gynecol Oncol 2004; 93(1): 204-8. doi: 10.1016/j.ygyno.2003.12.029.
- Arend R, Bagaria M, Lewin SN, Sun X, Deutsch I, Burke WM, et al. Long-term outcome and natural history of uterine adenosarcomas. Gynecol Oncol 2010; 119(2):305-8. doi: 10.1016/j.ygyno.2010.07.001.
- Nathenson MJ, Ravi V, Fleming N, Wang WL, Conley A. Uterine adenosarcoma: A review. Curr Oncol Rep 2016; 18(11):68. doi: 10.1007/s11912-016-0552-7.
- McCluggage WG. My approach to the interpretation of endometrial biopsies and curettings. J Clin Pathol 2006; 59(8):801-12. doi: 10.1136/jcp.2005.029702.
- McCluggage WG. Mullerian adenosarcoma of the female genital tract. Adv Anat Pathol 2010; 17(2):122-9. doi: 10.1097/PAP.0b013e3181cfe732.
- Carroll A, Ramirez PT, Westin SN, Soliman PT, Munsell MF, Nick AM, et al. Uterine adenosarcoma: an analysis on management, outcomes, and risk factors for recurrence. Gynecol Oncol 2014; 135(3):455-61. doi: 10.1016/j.ygyno. 2014.10.022.
- Nathenson MJ, Conley AP. Prognostic factors for uterine adenosarcoma: A review. Expert Rev Anticancer Ther 2018; 18(11):1093-100. doi: 10.1080/14737140.2018.1518136.