The Potential Utility of Lead Compound, E18, in Inhibiting Docking of SARS-CoV-2 by inhibiting ACE2-B0AT1 Complex
By Faiq Amin, Sahar Zuberi, Syeda Sadia FatimaAffiliations
doi: 10.29271/jcpsp.2022.08.1096Sir,
The novel coronavirus (nCoV) infects a host by docking to the angiotensin-converting enzyme 2 (ACE2) receptor. This binding of trimeric spike glycoprotein (S) located in the outer envelope of the virion to ACE2 receptors in the host leads to viral entry resulting in cleavage of the trimeric S into two subunits, S1 and S2. The receptor binding domain (RBD) on S1 binds to the peptidase domain (PD) on the host ACE2 receptor while S2 facilitates membrane fusion. Binding of S1 to ACE2 receptor exposes host proteases to a cleavage site on S2. This cleavage is essential for membrane fusion. It is interesting to note that in comparison to the SARS-CoV, this binding is 10-20 times stronger with SARS CoV2.1 Once in the cytoplasm, the viral +ssRNA enters into the genome, it functions as an mRNA in the cytoplasm and starts to replicate. During the entry of the virus in the host cell, the antigen is presented to antigen-presenting cells which activate the immune system, leading to a cytokine storm.2
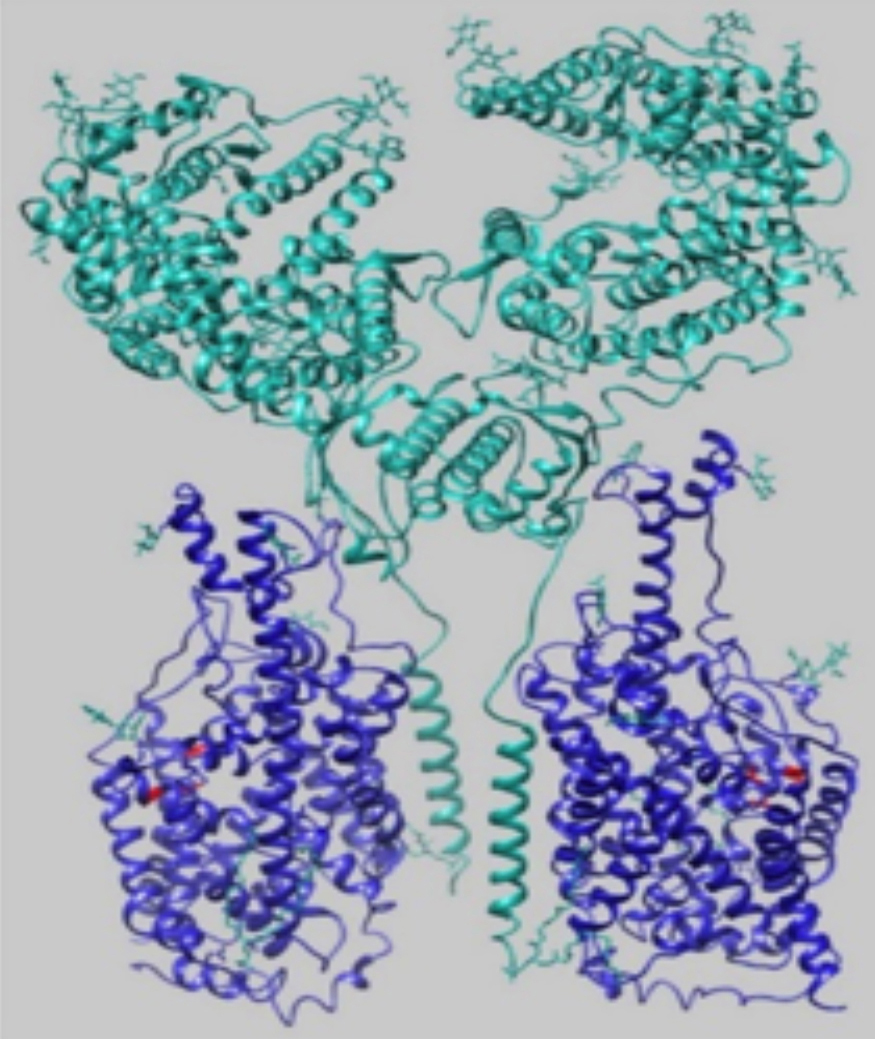 Figure 1: 3-D structure of (ACE2-B0AT1)2. ACE2 subunit (green) and B0AT1 subunit (blue) form heterodimers. Two of these heterodimers dimerise to form this superstructure (1).
Figure 1: 3-D structure of (ACE2-B0AT1)2. ACE2 subunit (green) and B0AT1 subunit (blue) form heterodimers. Two of these heterodimers dimerise to form this superstructure (1).
Recently, ACE2 has been found to serve as a chaperone for a means of transport across an amino acid transporter, B0AT1 (or SLC6A19).3 ACE2 and B0AT1 form a heterodimer, (ACE2-B0AT1). These heterodimers homodimerise to form a superstructure (ACE2-B0AT1)2 as depicted in Figure 1. This 3-D structure suggests that B0AT1 stabilizes ACE2 for efficient binding of S1.
ACE2-B0AT1 complexes have been identified in the kidney, intestines, and lungs; all vital systems, where nCoV gains host access. ACE2-B0AT1 complexes primarily transport neutral amino acids into the cells, thus reduced activity of B0AT1 can lead to excessive excretion of amino acids.4
In a recent study, repurposing of Nimesulide has been suggested to target the regulatory effect of B0AT1 on the binding of ACE2 receptor with RBD.5 However, due to its hepatotoxic effects, its use remains controversial. We propose using a lead compound, E18, which has structural homology with B0AT1. In intestinal cells, it acts as a potent inhibitor of B0AT1 by competitively inhibiting substrate binding by interfering with cation-pi interaction with sodium ions.6 It will inhibit the entry of SARS-CoV2 through ACE2 receptors by blocking the ACE-B0AT1 complexes. Symptoms that can result from this blockage are potential side effects similar to that of Hartnup disease. We propose administering aerosolised E18 by nebulisation to ensure efficient delivery to the lung tissue. Moreover, this will avoid its deleterious effects on other organs that express B0AT1 by minimally entering the bloodstream.
There is still a paucity of standardised treatment options for the treatment of COVID-19. More importantly, once pneumonia sets in, it can spread to all parts of the lungs leading to possibly fatal outcomes. Our hypothesis for the treatment of COVID-19 pneumonia revolves around the nebulised (inhaled) E18 compound that will function as an inhibitor of B0AT1 protein that is central in stabilising the ACE2-S protein complexes. The next step will be to perform docking studies of this compound on the ACE2-S complex. Laboratory work would then be done on making a nebulisable form of E18.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
FA, SZ, SSF: Conceptualised and wrote manuscript.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020; 367 (6483):1260-3. doi: 10.1126/science.abb2507.
- Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 2020; 10(2):102-8. doi: 10.1016/j.jpha.2020.03.001.
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020; 367(6485):1444-8. doi: 10.1126/ science.abb2762.
- Broer S. The role of the neutral amino acid transporter B0AT1 (SLC6A19) in Hartnup disorder and protein nutrition. IUBMB Life 2009; 61(6):591-9. doi: 10.1002/iub.210.
- Scalise M, Indiveri C. Repurposing nimesulide, a potent inhibitor of the B0AT1 Subunit of the SARS-CoV-2 receptor, as a Therapeutic Adjuvant of COVID-19. SLAS Discov 2020; 25(10):1171-3. doi: 10.1177/2472555220934421.
- Yadav A, Shah N, Tiwari PK, Javed K, Cheng Q, Aidhen IS, et al. Novel Chemical scaffolds to inhibit the neutral amino acid transporter B0AT1 (SLC6A19), a potential target to treat metabolic diseases. Front Pharmacol 2020; 11:140. doi: 10.3389/fphar.2020.00140.