The Efficiency of the HALP Score and the Modified HALP Score in Predicting Mortality in Patients with Acute Heart Failure Presenting to the Emergency Department
By Salih Kocaoglu, Tufan AlatliAffiliations
doi: 10.29271/jcpsp.2022.06.706ABSTRACT
Objective: To investigate the relationship between the HALP score (haemoglobin, albumin, lymphocyte, and platelet), the modified HALP (m-HALP) score, and prognosis in patients presenting to the emergency department (ED) with acute heart failure (AHF).
Study Design: A Descriptive study.
Place and Duration of Study: The Emergency Department of Balikesir University Hospital, Turkey, between January 2019 and September 2021.
Methodology: Patients diagnosed with AHF were divided into two groups, namely survivors and non-survivors. Both groups were compared in terms of HALP, m-HALP, PLT, NLR and PLR values ROC curve analysis was performed to evaluate their diagnostic performances in discriminating between one-week and three-month mortality. Youden J index was used to obtain the optimal cut-off value.
Results: The mean age of 101 patients included in the study was 73.15±10.19 years, with 51.5% (n=52) females, and 48.5% (n=49) males. The 1-week and 3-month mortality rates were 11.9% and 38.6%, respectively. For 1-week (p=0.018) and 3-month (p=0.006) mortality, platelet was significantly higher in the survivor group than in the non-survivor group. The m-HALP score and the NLR were found to be good predictors for 3-month mortality (p=0.002 and 0.016 respectively). The optimal cut-off values of m-HALP score, the platelet, and the NLR in predicting 3-month mortality were found as ≤1081723.5, ≤217, and >8.4. The area under curve (AUC) values were found as 0.687, 0.663, and 0.643, respectively. The sensitivity of the m-HALP score, the platelet, and the NLR were 76.92, 66.67, and 46.15, and the specificity values were 56.45, 67.74, and 79.03, respectively.
Conclusion: The m-HALP score was found to be a potential independent prognostic index for patients with AHF. The classical HALP score was not adequate to predict early and late prognosis. In addition, thrombocytopenia and increased NLR were associated with increased mortality in patients with AHF.
Key Words: Acute heart failure (AHF), HALP score, m-HALP score, Neutrophil lymphocyte ratio (NLR), Platelet lymphocyte ratio (PLR), Platelet (PLT).
INTRODUCTION
Acute heart failure (AHF) is a clinical condition requiring urgent treatment, which is characterized by worsening of symptoms and signs and an increase in dyspnea in patients with heart failure (HF). Its prevalence in the adult population is 2%, and it leads to an important economic burden in the global health system.1
AHF constitutes a significant portion of patients presenting to emergency services (ED) with acute dyspnea and is a serious cause of mortality and morbidity. The in-hospital mortality rate is 4-7%, and the rate of re-hospitalisation after discharge is 25-30%.2 The distinction between low-risk and high-risk patients can prevent unnecessary hospitalisations and reduce the economic burden. Therefore, biomarkers and scoring systems that can predict poor prognosis in patients with AHF can benefit patient management and reduce mortality rates.
The HALP score, which has been developed recently and consists of haemoglobin, albumin, lymphocytes, and platelets, has been used as a prognostic marker in various diseases. It has been shown to be a good predictor of mortality, especially in stomach, bladder, prostate, and kidney cancers and patients with stroke.3-7 The HALP score is especially effective on the geriatric patient population and reflects the patients’ general nutritional status and systemic inflammation. Anaemia and hypoalbuminemia are indicators of malnutrition. Anaemia is also known to increase cardiac decompensation.8 Lymphocytes play a key role in inflammation. Lymphopenia is associated with lower survival in patients with HF.9 Since elevated platelet increases the risk for thromboembolism and atherosclerotic lesions, it has been shown to be associated with mortality.10 In other words, previous studies on HALP scores have shown that low haemoglobin, albumin, lymphocyte ratios, and high platelet ratios are associated with poor patient prognosis. However, according to our hypothesis, low platelet is a more significant indicator of mortality. Therefore, the authors developed the modified HALP (m-HALP) score and compared it to the classical HALP score in patients with AHF.
Although the HALP score is used to predict prognosis in various diseases, it was first studied in patients with AHF in the literature. This study was conducted to evaluate and compare the predictive power of the HALP score and the newly developed m-HALP score on 1-week and 3-month mortality in patients admitted to the emergency department with AHF.
METHODOLOGY
This study was carried out in Balikesir University Hospital, Balikesir, Turkey retrospectively. It was approved by the Clinical Research Ethics Committee (Date: November 3, 2021; Issue: 2021/248). Patients diagnosed with AHF in the ED between January 2019 and September 2021 were retrospectively searched from hospital records. Patients who were under the age of 18, whose survival could not be followed up, and whose laboratory data were missing were excluded from the study. The diagnosis of AHF was made by the on-call emergency specialist and confirmed by the consultant cardiologist. Up-to-date ESC guidelines were used when making the decision.
Haemoglobin, albumin, lymphocyte, platelet, and neutrophil values of the patients were recorded at the time of admission. Blood counts and biochemical parameters were tested using an automated analyzer (Beckman Coulter Hematology Analyzer LH780, Beckman Coulter Chemistry Analyzer AU680). After that, the HALP score, the m-HALP score, the neutrophil-lymphocyte ratio (NLR), and the platelet lymphocyte ratio (PLR) were calculated with these values. The HALP score was calculated by using the haemoglobin (g/L) × albumin (g/L) × lymphocyte count (/L) / platelet count (/L) method. The m-HALP score was calculated by using the haemoglobin (g/L) × albumin (g/L) × lymphocyte count (/L) × platelet count (/L) method. The NLR was obtained by dividing the neutrophil count by the lymphocyte count, while the PLR was obtained by dividing the platelet count by the lymphocyte count.
Poor outcomes were determined as 1-week and 3-month mortality status of the patients. Data about the survival status of the patients were obtained from the patient files and hospital database. Patients whose data could not be obtained were contacted by phone. Seven patients whose 1-week and 3-month mortality data could not be reached by either of the methods were excluded from the study.
Shapiro-Wilk test was used to test the normality of the quantitative variables. Continuous variables with normal distribution were expressed as mean ± standard deviation values. Non-normal variables were expressed as median (1st quantile-3rd quantile) values. Comparisons between two independent groups were performed with independent samples t-test for numerical variables, such as age, haemoglobin, albumin, lymphocytes, neutrophils, PLT, NLR, PLR, HALP score and m-HALP score when they followed a normal distribution in two groups, otherwise Mann-Whitney U-test was used. Pearson chi-square test was used to make a gender comparison between survivors and non-survivors, n and % values were given for gender. Receiver operating characteristics (ROC) curve analysis was performed to evaluate the diagnostic performances of HALP score, m-HALP score, PLT, NLR, and PLR in discriminating 1-week and 3-month survivors and non-survivors. Youden J index was used to obtain the optimal cut-off value, and related sensitivity, specificity, positive predictive, and negative predictive values were given. p<0.05 was considered statistically significant. Statistical analyses were performed on the IBM SPSS Statistics version 23.0 (IBM Corp., USA) and MedCalc version 12.3.0.0.
Table I: Comparison of patient characteristics in terms of one-week mortality.
|
Variables |
Survivor (n=89) |
Non-survivor (n=12) |
p-value |
|
Age (years)* |
73.37±10.18 |
71.50±10.49 |
0.553 |
|
Gender# Female Male |
44 (49.4) 45 (50.6) |
8 (66.7) 4 (33.3) |
0.262 |
|
Hb (g/L)* |
113.56±18.29 |
124.42±27.09 |
0.073 |
|
Albumin (g/L)* |
34.00 (31.00-38.00) |
32.00 (30.25-36.25) |
0.357 |
|
Lym (109/L)* |
1.10 (0.70-1.60) |
1.10 (0.60-1.53) |
0.781 |
|
Neu (109/L)* |
7.00 (5.35-8.70) |
7.20 (6.13-8.55) |
0.407 |
|
PLT (109/L)* |
242.00 (188.50-316.00) |
177.00 (133.25-216.75) |
0.018 |
|
NLR (neu/lym)* |
5.25 (4.09-9.00) |
7.25 (4.23-13.30) |
0.502 |
|
PLR (plt/lym)* |
207.69 (141.22-362.50) |
178.19 (108.19-305.47) |
0.236 |
|
HALP score* |
18.23 (9.88-28.98) |
26.03 (11.51-35.12) |
0.204 |
|
m-HALP score* |
1023264.00 (577244.20-1796784.00) |
642414.30 (434061.90-1875019.88) |
0.257 |
|
#Data given as mean ± standard deviation; independent samples t-test was applied, *Data given as median (Q1-Q3); Mann-Whitney U-test was applied, and Data given as n (%); Pearson Chi-square test was applied. |
|||
Table II: Comparison of patient characteristics in terms of three-month mortality.
|
Variables |
Survivor (n=62) |
Non-survivor (n=39) |
p-value |
|
Age (years) # |
72.61±10.92 |
74.00±8.96 |
0.508 |
|
Gender & Female Male |
32 (51.6) 30 (48.4) |
20 (51.3) 19 (48.7) |
0.974 |
|
Hb (g/L)# |
112.97±17.36 |
117.85±22.82 |
0.227 |
|
Albumin (g/L)* |
34.00 (31.00-39.00) |
33.00 (30.00-36.00) |
0.063 |
|
Lym (109/L)* |
1.25 (0.78-1.73) |
0.90 (0.60-1.20) |
0.020 |
|
Neu (109/L)* |
6.60 (5.38-8.60) |
7.20 (5.70-8.90) |
0.294 |
|
PLT (109/L)* |
266.00 (201.25-312.75) |
202.00 (150.00-253.00) |
0.006 |
|
NLR (neu/lym)* |
4.95 (3.88-7.81) |
6.67 (4.73-14.60) |
0.016 |
|
PLR (plt/lym)* |
198.80 (135.66-353.54) |
215.00 (139.00-362.50) |
0.706 |
|
HALP score* |
19.25 (10.02-31.70) |
16.69 (10.14-30.33) |
0.630 |
|
m-HALP score* |
1325791.75 (663724.25-1981335.20) |
716086.80 (396264.00-1081723.50) |
0.002 |
|
#Data given as mean ± standard deviation; independent samples t-test was applied. *Data given as median (Q1-Q3); Mann-Whitney U-test was applied. Data given as n (%); Pearson Chi-square test was applied. |
|||
RESULTS
A total of 101 patients were enrolled in the study. The mean age of the patients was 73.15±10.19 years. There were 52 (51.5%) female and 49 (48.5) male patients. One-week and three-month mortality rates were 11.9% (n=12) and 38.6% (n=39), respectively. When the authors compared the variables between survivals and non-survivals, PLT was significantly higher in the survival group than the non-survival group for one-week and three-month mortality. Also, there was a significant difference in terms of lymphocytes, PLT, NLR, and m-HALP scores between the two groups for three-month mortality (Tables I,II, Figures 1-3).
The diagnostic performances of HALP score, m-HALP score, PLT, NLR and PLR were examined for predicting one-week and three-month mortality. For one-week mortality, only PLT showed a significant diagnostic performance with an optimal cut-off value of ≤217 (AUC= 0.71, p=0.009). HALP score, m-HALP score, NLR and PLR didn’t show significant diagnostic performances. For three-month mortality, m-HALP score, PLT and NLR showed significant diagnostic performances with optimal cut-off values of ≤1081723.5, ≤217 and >8.4, respectively (Table III). When the authors compared the diagnostic performances of the m-HALP score, PLT and NLR for three-month mortality, we couldn’t find a significant difference in pair wise comparisons (m-HALP vs. PLT: p=0.677; m-HALP vs. NLR: p=0.372, PLT vs. NLR: p=0.807, Figure 4).
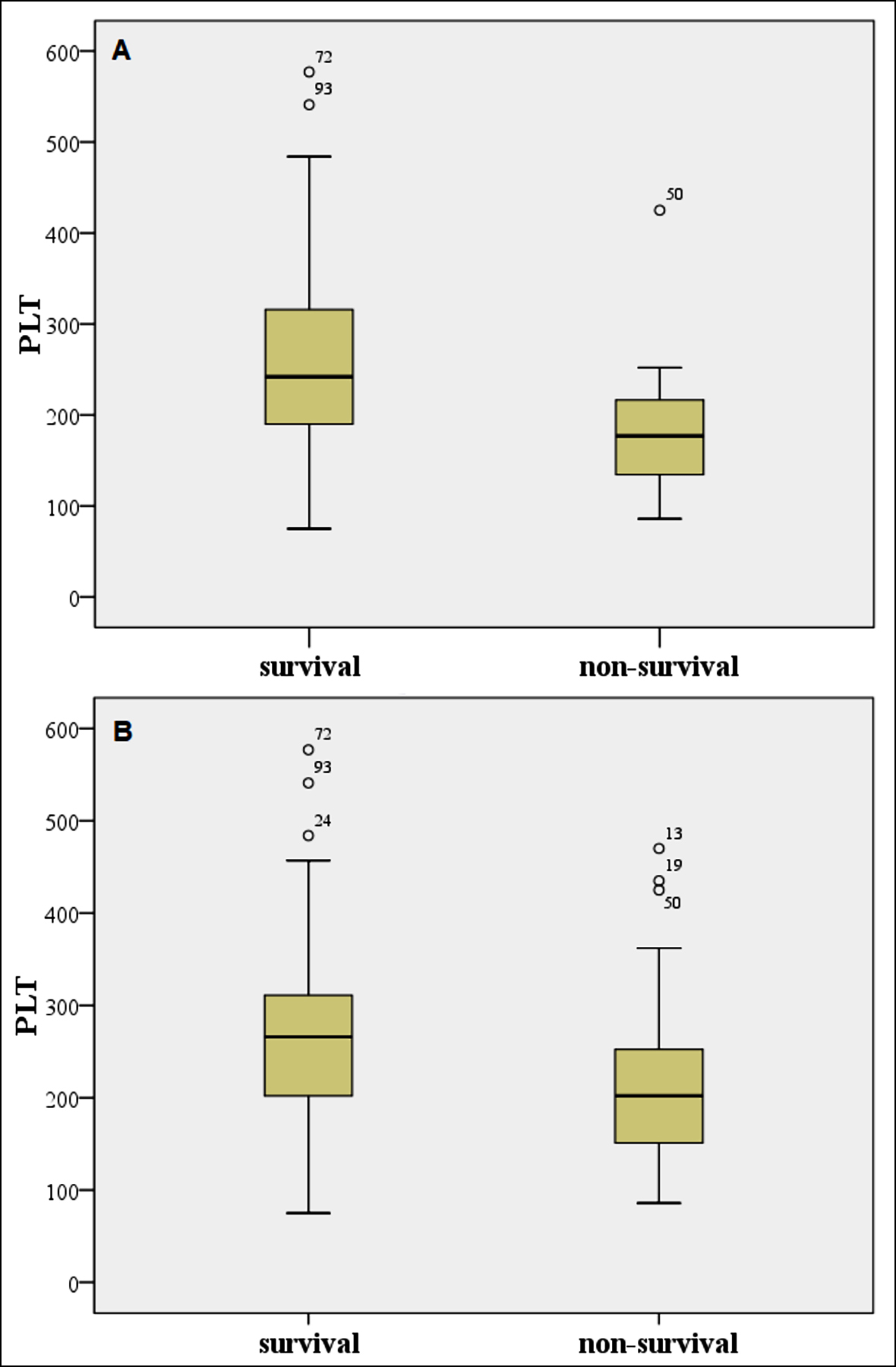 Figure 1: Box-plots for PLT for (A) one-week survivals and non-survivals, (B) three-month survivals and non-survivals; Outliers and extreme values are marked with ° and *, respectively.
Figure 1: Box-plots for PLT for (A) one-week survivals and non-survivals, (B) three-month survivals and non-survivals; Outliers and extreme values are marked with ° and *, respectively.
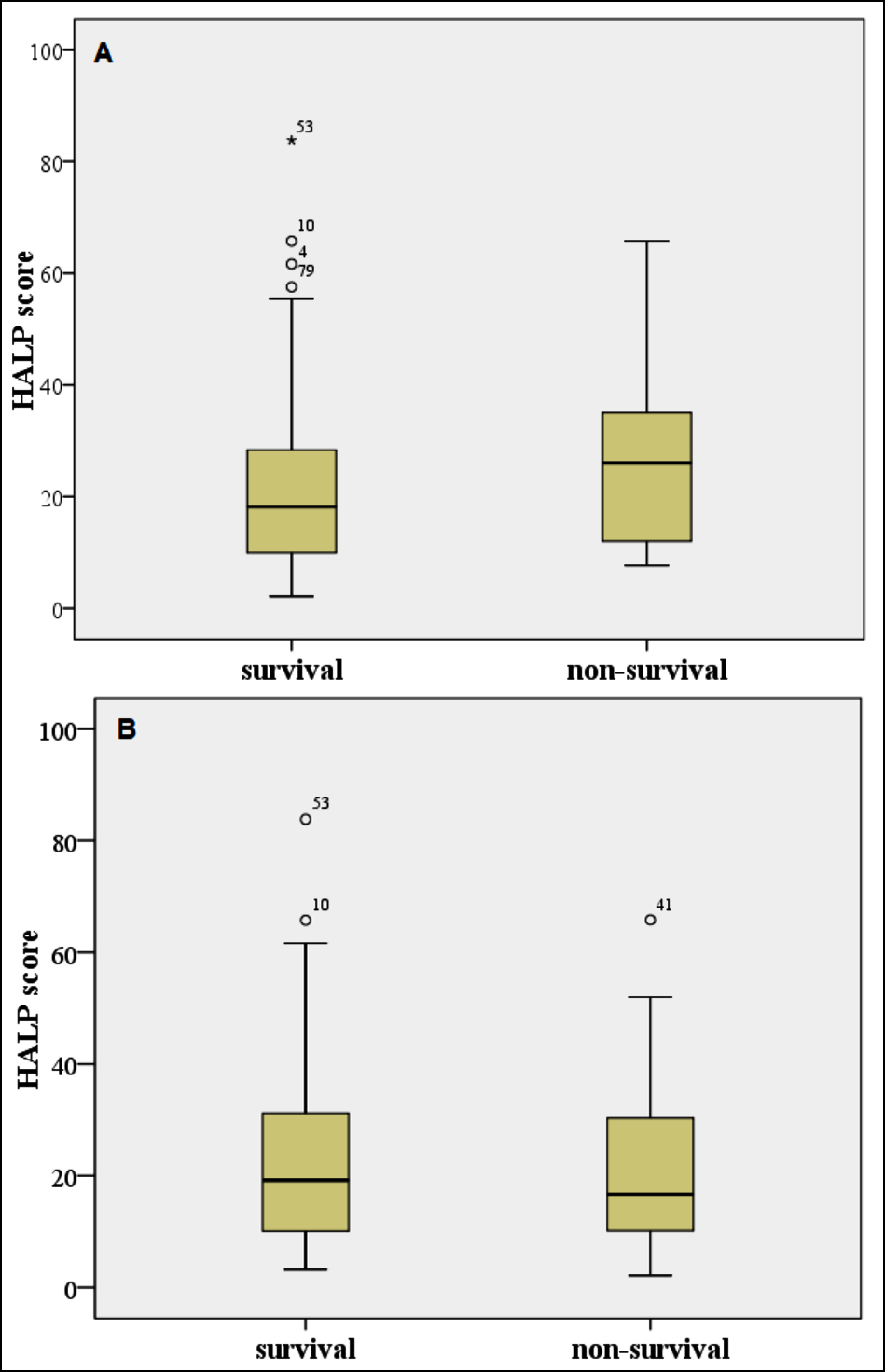 Figure 2: Box-plots for HALP score for (A) one-week survivals and non-survivals, (B) three-month survivals and non-survivals; Outliers and extreme values are marked with ° and *, respectively.
Figure 2: Box-plots for HALP score for (A) one-week survivals and non-survivals, (B) three-month survivals and non-survivals; Outliers and extreme values are marked with ° and *, respectively.
DISCUSSION
In this study, the predictive power of the newly developed HALP score, which has been shown to be used as a prognostic marker in various diseases, and the m-HALP score, which the authors derived from it, on mortality in patients with AHF was investigated. In the study, the m-HALP score was shown to be a significant predictor of 3-month mortality in patients with AHF. It was understood that an increased m-HALP score was an indicator of a good prognosis. In addition, PLT, lymphocyte, and NLR values were found to give significant results for 3-month mortality. Low PLT alone was associated with increased 1-week and 3-month mortality in AHF. The classical HALP score was shown to be not associated with prognosis in patients with AHF.
The HALP score was first used by Chen et al. to predict prognosis in gastric cancer.5 It was later used to predict prognosis in prostate cancer, bladder cancer, lung cancer, colorectal cancer, and patients with ischemic stroke.3,6,7,11,12 As is known, while haemoglobin and albumin levels indicate the nutritional status of the body, lymphocytes, and platelets are associated with immune status. Studies have shown that a low HALP score is associated with a poor prognosis. In a recent study by Ferhat et al. on patients who underwent sleeve gastrectomy, it was shown that patients with high HALP scores lost more weight and their laboratory parameters improved significantly.13 In this study, we examined the HALP score and m-HALP score in patients with AHF for the first time in the literature.
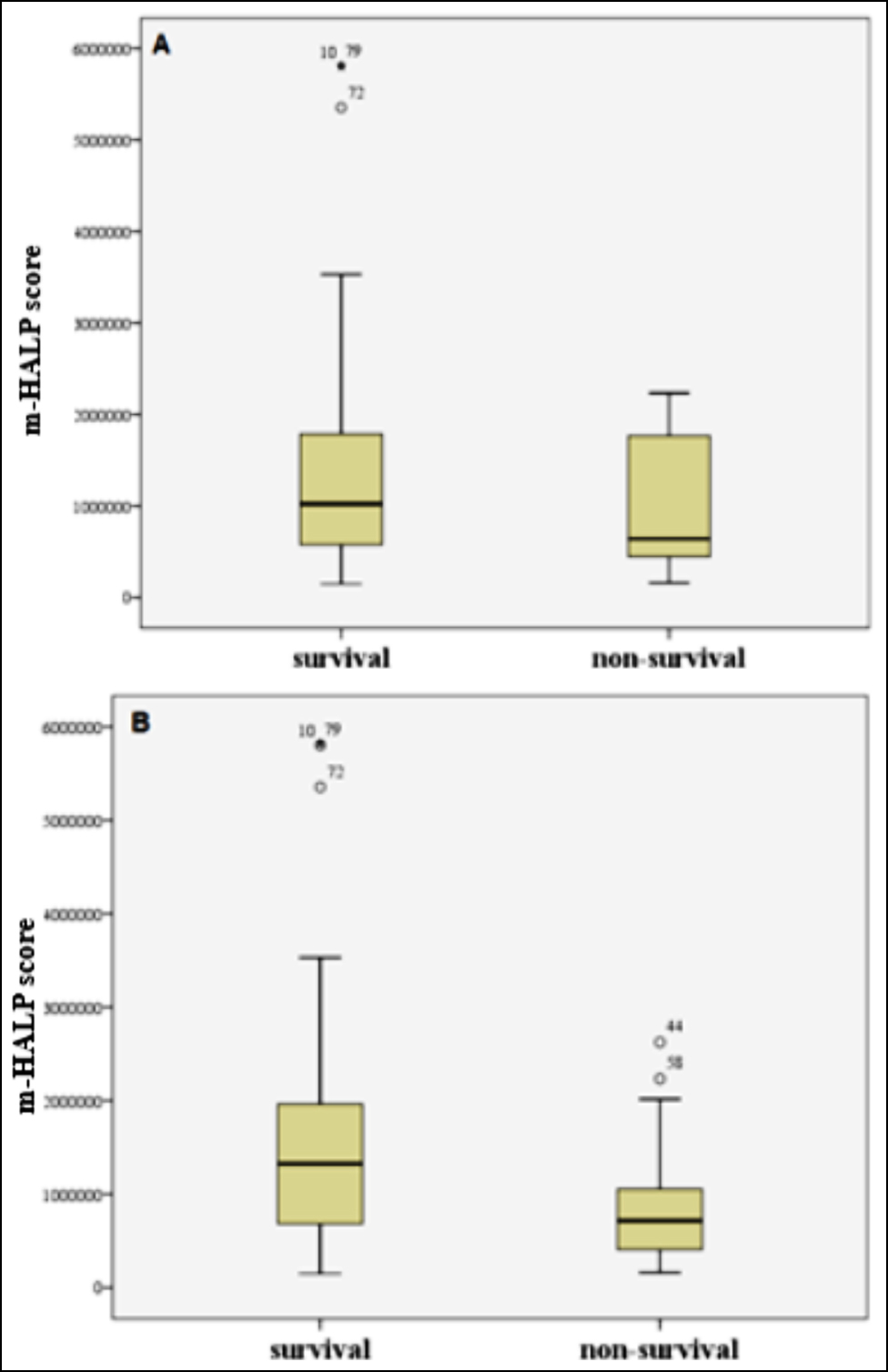 Figure 3: Box-plots for m-HALP score for A) one-week survivals and non-survivals, B) three-month survivals and non-survivals; Outliers and extreme values are marked with ° and *, respectively.
Figure 3: Box-plots for m-HALP score for A) one-week survivals and non-survivals, B) three-month survivals and non-survivals; Outliers and extreme values are marked with ° and *, respectively.
The first of the parameters that make up the HALP score is the haemoglobin value. It is known that anaemia triggers cardiac decompensation in patients with HF. In addition, some intervention studies have shown that correction of anaemia in patients with congestive heart failure (CHF) improves the quality of life.8,14 Torrijos et al. showed that haemoglobin level below 12g/dL was associated with all-cause death, cardiac death, and re-hospitalisation for HF in patients with AHF.15 Mozaffarian et al. stated that anaemia was an independent risk factor for mortality in patients with severe heart failure.16 Many studies in the literature present similar results. Unlike the literature, haemoglobin did not show a statistically significant difference for 1-week and 3-month mortality in this study.
Table III: ROC curve analysis results for HALP score, m-HALP score, NLR and PLR.
|
Accuracy index |
HALP score |
m-HALP score |
PLT |
NLR |
PLR |
|
One-week mortality |
|||||
|
AUC |
- |
- |
0.711 |
- |
- |
|
p-value |
0.187 |
0.289 |
0.009 |
0.521 |
0.219 |
|
cut-off value |
- |
- |
≤217 |
- |
- |
|
Youden J index |
- |
- |
0.429 |
- |
- |
|
Sensitivity (95% CI) |
- |
- |
83.33 (51.6-97.9) |
- |
- |
|
Specificity (95% CI) |
- |
- |
59.55 (48.6-69.8) |
- |
- |
|
Three-month mortality |
|||||
|
AUC |
- |
0.687 |
0.663 |
0.643 |
- |
|
p-value |
0.628 |
<0.001 |
0.006 |
0.013 |
0.709 |
|
cut-off value |
- |
≤1081723.5 |
≤217 |
>8.4 |
- |
|
Youden J index |
- |
0.334 |
0.344 |
0.252 |
- |
|
Sensitivity (95% CI) |
- |
76.92 (60.7 – 88.9) |
66.67 49.8 - 80.9 |
46.15 (30.1 - 62.8) |
- |
|
Specificity (95% CI) |
- |
56.45 (43.3 – 69.0) |
67.74 (54.7 - 79.1) |
79.03 (66.8 - 88.3) |
- |
|
AUC: Area under the curve, CI: Confidence interval. |
|||||
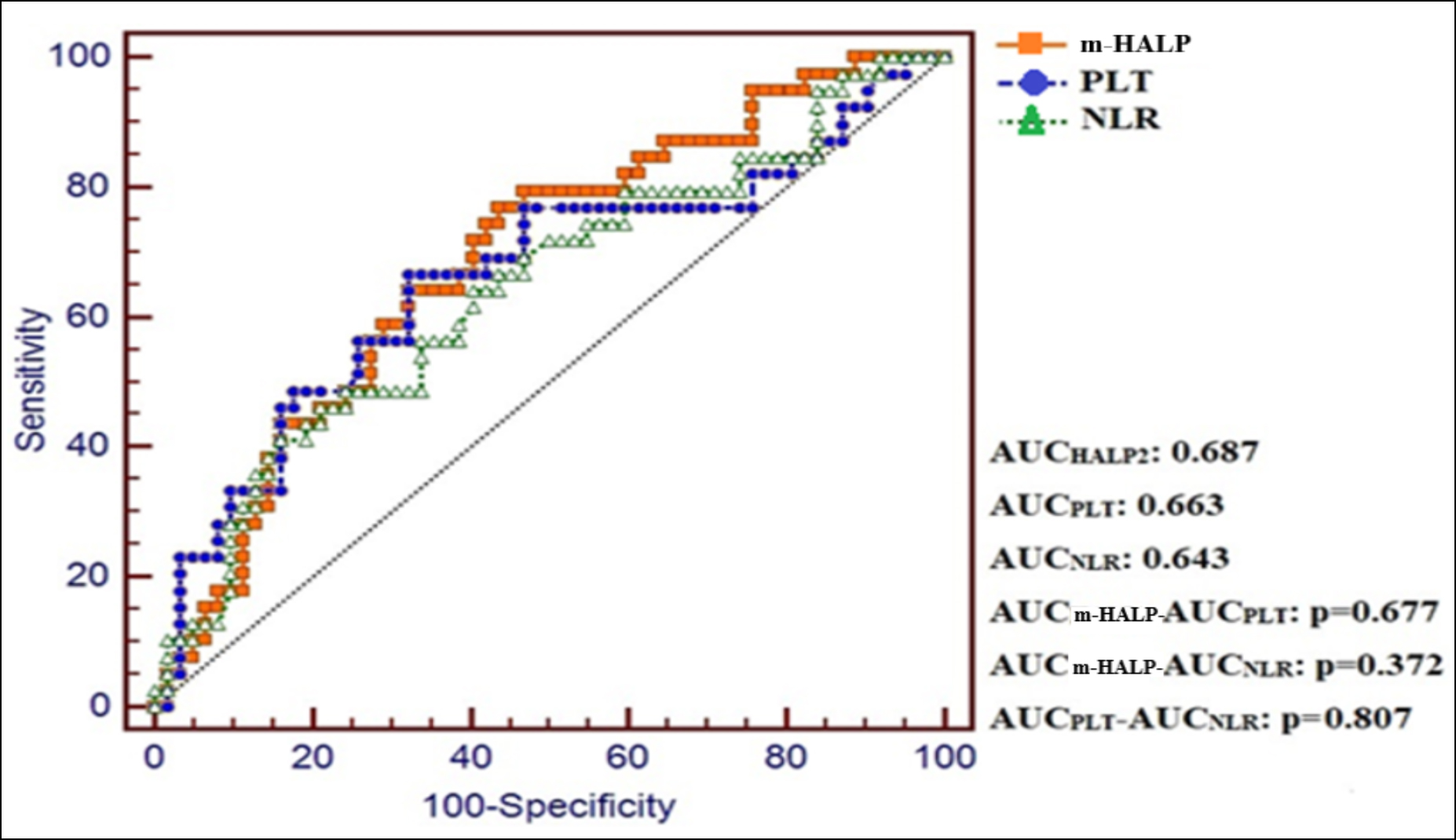 Figure 4: ROC curves for m-HALP score, PLT and NLR for predicting three-month mortality.
Figure 4: ROC curves for m-HALP score, PLT and NLR for predicting three-month mortality.
Serum albumin, the second component of the score, acts as the main protein in the intravascular spaces of the human body. It is also known as a negative acute-phase reactant. Previous clinical studies have shown that albumin level is a reliable marker for malnutrition, inflammation, and comorbidity in patients with HF.17-19 In this study, the mean serum albumin level of patients with AHF who survived was found to be higher than the level of those who did not, but there was no statistically significant difference.
It is known that lymphopenia is an independent prognostic marker associated with low survival in patients with HF.9,20 Cortisol secretion occurs in the hypothalamic-pituitary-adrenal axis following the stress factor associated with AHF, and this hormone causes a decrease in lymphocyte concentrations. Decreased lymphocyte levels, on the other hand, cause poor outcomes in patients with AHF. In this study, the lymphocyte level, which is the third parameter, was found to be statistically significantly higher in patients who survived.
The relationship between platelet level and the prognosis of AHF has not been clearly clarified yet. There are conflicting studies in the literature. Some studies suggest that platelet elevation increases the risk for thromboembolism and atherosclerotic lesions and that it has a positive correlation with related deaths due to AHF.21,22 However, it is well known from the intensive care literature that low or decreased PLT count is associated with worsening disease status and is an independent predictor of mortality.23 In their study on 1907 patients with heart failure and low ejection fraction, Mucadidi et al. showed that the degree of thrombocytopenia was associated with mortality.24 Similarly, Yamaguchi et al. examined 425 patients with AHF and showed that low platelets were associated with an increased risk for all-cause deaths and readmission due to HF.25 In this study, PLT alone was found to be a significant predictor for 1-week and 3-month mortality. It was determined that PLT levels in the non-survivor patient group were lower than the levels in the survivor patient group.
It is evident in this study that thrombocytopenia is a significant biomarker of mortality in patients with AHF. Therefore, the HALP score was rearranged as the m-HALP score, and the PLT count was added to the numerator. The relationship of the m-HALP score and the classical HALP score with mortality in patients with AHF was examined. The m-HALP score was a significant predictor of 3-month mortality in patients with AHF. The classical HALP score, on the other hand, was not significant for 1-week and 3-month mortality. Optimal cut-off values for the m-HALP score, the PLT, and the NLR, which were found to be effective in predicting 3-month mortality in patients with AHF, were ≤1081723.5, ≤217, and >8.4, respectively. When their diagnostic performances was compared by using ROC analysis, it was seen that there was no significant difference between them.
This study has some limitations. First of all, it is a retrospective study, and our data sample is limited. Our population selection has the potential to be biased, as it includes patients followed up by a single centre and using a similar treatment method. Patients whose survival data could not be obtained were excluded from the study. The authors think that our study should be supported by additional multicenter studies to be conducted with larger patient groups.
CONCLUSION
While the m-HALP score was found to be a significant parameter in predicting 3-month mortality in patients with AHF, the classical HALP score was an ineffective parameter to predict early and late prognosis. The m-HALP score can be used easily and inexpensively in clinical practice in patients with AHF to follow up treatment response and patient survival. In addition, thrombocytopenia and increased NLR are good predictors of mortality in AHF patients.
ETHICAL APPROVAL:
Ethics Committee approval was obtained from the Ethics Committee of the Faculty of Medicine, Balikesir University (Date: November 3, 2021; Issue: 2021/248).
PATIENTS’ CONSENT:
Since the study included a retrospective archive search, patient consent was not necessary.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTIONS
SK: Conceived and designed the study, data collection, paper write-up, and the compilation of the results.
TA: Statistic analyses, data collection, discussion, the compilation of the results, and proofreading.
REFERENCES
- Kurmani S, Squire I. Acute heart failure: Definition, classification and epidemiology. Curr Heart Fail Rep 2017; 14(5):385-392. doi: 10.1007/s11897-017-0351-y.
- Farmakis D, Parissis J, Filippatos G. Acute heart failure: Epidemiology, classification and pathophysiology. In: Tubaro M, Vranckx P, Price S, Vrints C, Eds. The ESC textbook of intensive and acute cardiovascular care, 2nd ed. Oxford; Oxford University Press; 2015: p. 459-69.
- Guo Y, Shi D, Zhang J, Mao S, Wang L, Zhang W, et al. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is a novel significant prognostic factor for patients with metastatic prostate cancer undergoing cytoreductive radical prostatectomy. J Cancer 2019; 10(1):81-91. doi: 10.7150/jca.27210.
- Peng D, Zhang CJ, Tang Q, Zhang L, Yang KW, Yu XT, et al. Prognostic significance of the combination of preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) in patients with renal cell carcinoma after nephrectomy. BMC Urol 2018; 18(1):20. doi: 10.1186/s12894-018-0333-8.
- Chen XL, Xue L, Wang W, Chen HN, Zhang WH, Liu K, et al. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: A retrospective cohort study. Oncotarget 2015; 6(38):41370-382. doi: 10.18632/onco-target.5629.
- Jiang H, Li H, Li A, Tang E, Xu D, Chen Y, et al. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget 2016; 7(44):72076-083. doi: 10.18632/oncotarget.12271.
- Tian M, Li Y, Wang X, Tian X, Pei LL, Wang X, et al. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is associated with poor outcome of acute ischemic stroke. Front Neurol 2021; 11:610318. doi: 10.3389/fneur. 2020.610318.
- Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalisations. J Am Coll Cardiol 2000; 35(7):1737-44. doi: 10.1016/s0735-1097(00)00613-6.
- Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol 1997; 79(6):812-814. doi: 10.1016/s0002-9149(96)00878-8.
- Reininger AJ, Bernlochner I, Penz SM, Ravanat C, Smethurst P, Farndale RW, et al. A 2-step mechanism of arterial thrombus formation induced by human atherosclerotic plaques. J Am Coll Cardiol 2010; 55(11): 1147-58. doi: 10.1016/j.jacc.2009.11.051.
- Peng D, Zhang CJ, Gong YQ, Hao H, Guan B, Li XS, et al. Prognostic significance of HALP (hemoglobin, albumin, lymphocyte and platelet) in patients with bladder cancer after radical cystectomy. Sci Rep 2018; 8(1):794. doi: 10.1038/s41598-018-19146-y.
- Shen XB, Zhang YX, Wang W, Pan YY. The Hemoglobin, Albumin, Lymphocyte, and platelet (HALP) score in patients with small cell lung cancer before first-line treatment with etoposide and progression-free survival. Med Sci Monit 2019; 25:5630-9. doi: 10.12659/MSM. 917968.
- Cay F, Duran A. Predictive factors of success in sleeve gastrectomy: One-year follow-up and the significance of HALP score. J Coll Physicians Surg Pak 2021; 31(12): 1406-11. doi: 10.29271/jcpsp.2021.12.1406.
- Mancini DM, Katz SD, Lang CC, LaManca J, Hudaihed A, Androne AS. Effect of erythropoietin on exercise capacity in patients with moderate to severe chronic heart failure. Circulation 2003; 107(2):294-99. doi: 10.1161/01.cir. 0000044914.42696.6a.
- Sanchez-Torrijos J, Gudin-Uriel M, Nadal-Barange M, Jacas-Osborn V, Trigo-Bautista A, Gimenez-Alcalá M, et al. Valor pronóstico de las cifras de hemoglobina en el momento del alta en pacientes hospitalizados por insuficiencia cardiaca [Prognostic value of discharge hemoglobin level in patients hospitalised for acute heart failure]. Rev Esp Cardiol 2006; 59(12):1276-82. doi: 10.1157/13096601.
- Mozaffarian D, Nye R, Levy WC. Anemia predicts mortality in severe heart failure: the prospective randomised amlodipine survival evaluation (PRAISE). J Am Coll Cardiol 2003; 41(11):1933-9. doi: 10.1016/s0735-1097(03)00425-x.
- Polat N, Aydin M, Yildiz A, Acet H, Akil MA, Bilik MZ, et al. The prognostic significance of serum albumin in patients with acute decompensated systolic heart failure. Acta Cardiol 2014; 69(6):648-54. doi: 10.1080/ac.69.6.1000 007.
- Liu M, Chan CP, Yan BP, Zhang Q, Lam YY, Li RJ, et al. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2012; 14(1):39-44. doi: 10.1093/eurjhf/hfr154.
- Cinar T, Hayıroglu MI, Cicek V, Orhan AL. A simple parameter for long-term mortality in acute heart failure patients: Serum albumin. Acta Cardiol 2021; 76(1):106. doi: 10.1080/00015385.2019.1699280.
- Durmus E, Kivrak T, Gerin F, Sunbul M, Sari I, Erdogan O. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio are predictors of heart failure. Arq Bras Cardiol 2015; 105(6):606-13. doi: 10.5935/abc.20150126.
- Ye GL, Chen Q, Chen X, Liu YY, Yin TT, Meng QH, et al. The prognostic role of platelet-to-lymphocyte ratio in patients with acute heart failure: A cohort study. Sci Rep 2019; 9(1):10639. doi: 10.1038/s41598-019-47143-2.
- Pourafkari L, Wang CK, Tajlil A, Afshar AH, Schwartz M, Nader ND. Platelet-lymphocyte ratio in prediction of outcome of acute heart failure. Biomark Med 2018; 12(1):63-70. doi: 10.2217/bmm-2017-0193.
- Vanderschueren S, De Weerdt A, Malbrain M, Vankersschaever D, Frans E, Wilmer A, et al. Thrombocytopenia and prognosis in intensive care. Crit Care Med 2000; 28(6):1871-1876. doi: 10.1097/0000 3246-200006000-00031.
- Mojadidi MK, Galeas JN, Goodman-Meza D, Eshtehardi P, Msaouel P, Kelesidis I, et al. Thrombocytopaenia as a prognostic indicator in heart failure with reduced ejection fraction. Heart Lung Circ 2016; 25(6):568-75. doi: 10.1016/j.hlc.2015.11.010.
- Yamaguchi S, Abe M, Arakaki T, Arasaki O, Shimabukuro M. Incremental prognostic value of platelet count in patients with acute heart failure - a retrospective observational study. Circ J 2019; 83(3):576-83. doi: 10.1253/circj.CJ-18-0961.