Symptomatic Spinal Seeding Metastasis of a Low-grade Oligodendroglioma
By Emrah Keskin1, Hasan Ali Aydin1, Burak Bahadir2, Kenan Simsek1, Murat Kalayci1Affiliations
doi: 10.29271/jcpsp.2022.10.1347ABSTRACT
Extracranial metastases from primary brain tumours are mostly caused by high-grade tumours. Metastases from low-grade intracranial tumours are much rare and usually asymptomatic. We present a case of a symptomatic spinal cord compression with intradural extramedullary and diffuse leptomeningeal infiltration observed approximately 51 months after the first diagnosis of a 52-year male patient with WHO Grade 2 oligodendroglioma with temporoparietal localisation. This patient, who had the complaint of weakness in the lower extremity, was operated on due to a thoracic intradural extramedullary mass. The result of the pathological examination came out as WHO Grade 2 oligodendroglioma, and radiotherapy was planned for this seeding metastasis. The patient who experienced refractory seizures died before his radiotherapy treatment was completed. It should be kept in mind that spinal metastases may also be seen in low-grade intracranial tumours without malignant transformation as in the present case.
Key Words: Spinal seeding, Spinal metastases, Low-grade oligodendroglioma.
INTRODUCTION
Oligodendrogliomas (OG) refer to neuroepithelial tumours pathologically classified as grade II (low-grade) and anaplastic tumours with a worse prognosis classified as grade III (high grade) according to the 2016 World Health Organization (WHO) classification.1,2
Spinal metastases from OG are extremely rare and occur at different times in the course of the disease. In the literature, cases with spinal metastases have been reported within three months to nine years following the diagnosis of OG.3,4 Here, we present a case of an OG with spinal metastasis, which we attributed to tumour seeding approximately 51 months after the diagnosis of the brain tumour.
CASE REPORT
A 52-year male patient was being followed up with a mass filling the left temporoparietal and left ipsilateral lateral ventricular occipital horn, which was first diagnosed radiologically in 2012.
The lesion of the patient, who did not agree to a stereotactic biopsy, was assumed to be a low-grade glial tumour. It was decided to radiologically and clinically monitor the patient. In January 2014, he presented to the emergency department with a seizure. The patient was resistant to medical treatment and had frequent seizures, and therefore, he underwent surgery in a centre to which he was referred, and his pathology result was reported as WHO Grade II OG (Figure 1a, b). Radiotherapy was applied with a dose of 54 Gy in 27 fractions with 75 mg/m2 temozolomide postoperatively. The clinical target volume was created with a 1-cm margin for T1 enhancement and mass-like FLAIR imaging of the tumour volume, and the planning target volume was created with a 0.5-cm margin to the clinical tumour volume. The patient presented again in March 2016 with complaints of weakness and numbness in his right leg and low back pain, and his neurological examination revealed that his motor muscle strength on the right side was 3/5 in the distal and 2/5 in the proximal muscles. The contrast-enhanced thoracic MRI of the patient showed syringomyelia extending from T5 to T11, as well as linear, intense contrast enhancement of an intradural mass that compressed the two spinal corners at T9 and T12-L1 levels and the dura between the two lesions (Figure 1c, d). The patient exhibited severe neurological deficits and was taken to emergency surgery. Following total laminectomy of T12, the lesion fixed to the surrounding nerve intradural roots was subtotally excised. The pathology result of the lesion, which was immunohistochemically positive for S-100 and GFAP and negative for EMA, synaptophysin and pan-CK, was received as WHO Grade II OG (Figure 2). In the postoperative period, the patient’s neurological deficit improved, but six months later, he started to have complaints of widespread neck pain, back pain and weakness and numbness in the arms and legs. The cervico-thoracic MRI of the patient revealed extensive intense contrast enhancement starting from the anterior of the pons and medulla oblongata and extending to the thoracic region, dilatation of the syringomyelic cavity and compression of the spinal cord. Radiotherapy was planned for the entire spine with a dose of 30.6 Gy in 17 fractions with a boost dose of 14.4 Gy in 8 fractions to the tumour volumes. However, while the patient could not complete his radiotherapy treatment due to refractory and frequent seizures, he died 4 years and 3 months after his first diagnosis.
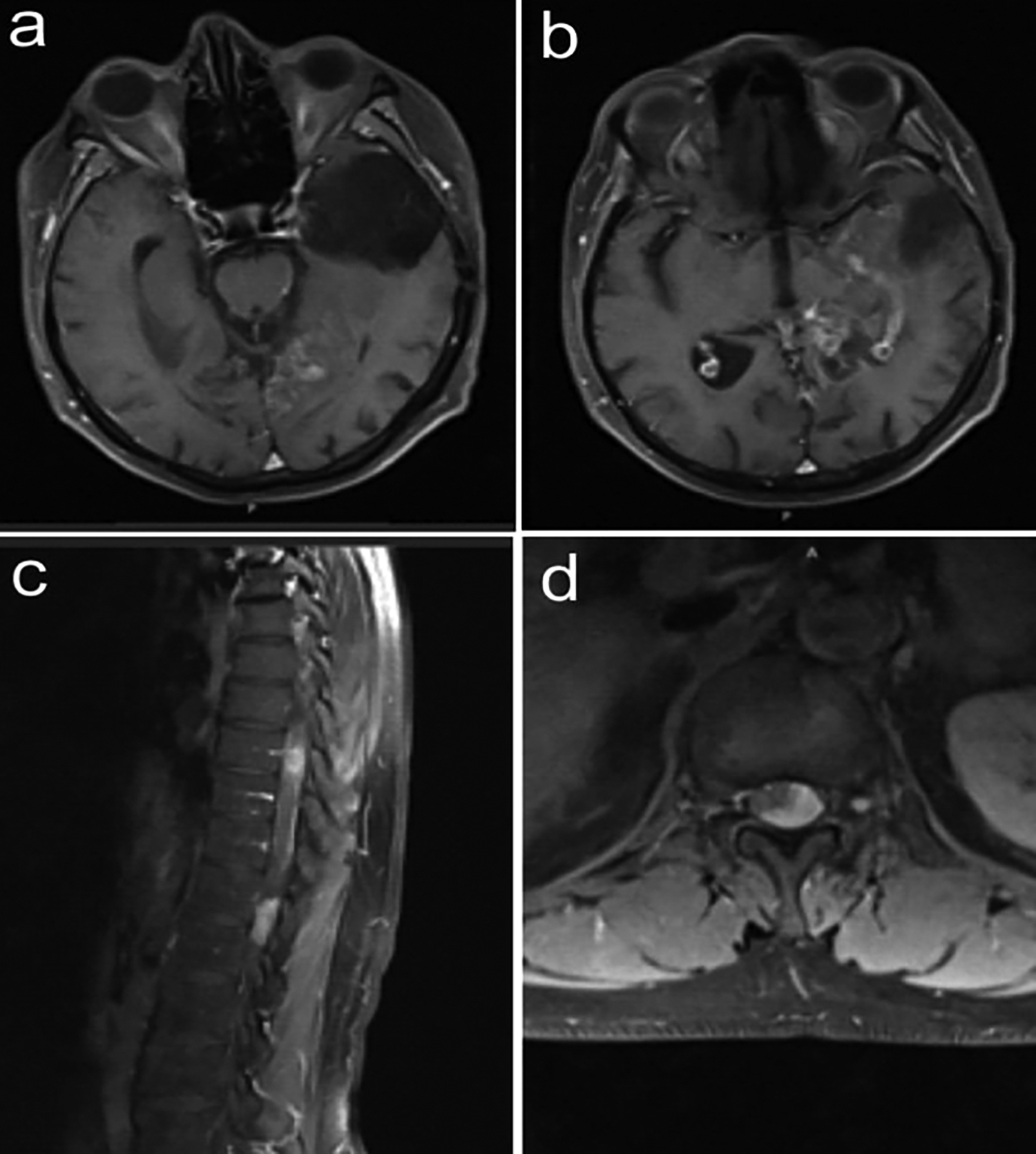 Figure 1: Postoperative axial contrast-enhanced brain MRI image showing the contrast involvement of the left temporal encephalomalacic area and left lateral ventricle posterior horn (a, b). Preoperative contrast-enhanced thoracic MRI images showing a spinal intradural-extramedullary mass at T9 and T12 levels in the sagittal plane and at T12 level in the axial plane (c, d).
Figure 1: Postoperative axial contrast-enhanced brain MRI image showing the contrast involvement of the left temporal encephalomalacic area and left lateral ventricle posterior horn (a, b). Preoperative contrast-enhanced thoracic MRI images showing a spinal intradural-extramedullary mass at T9 and T12 levels in the sagittal plane and at T12 level in the axial plane (c, d).
DISCUSSION
Although OGs are slow-growing and partially benign tumours with better survival than other gliomas, they tend to recur and lead to mortality. OGs rarely metastasize out of the central nervous system, similar to other neuroepithelial tumours.5 In literature reviews on extracranial metastasis of OGs, Singh et al. detected a total of 34 cases of distant metastases (bone/bone marrow, lymph nodes, internal organs, breast, scalp, parotid, and adrenal gland), including their own cases.5 Similar to the systemic spread of OGs, spinal metastases are rare but tend to invade the leptomeningeal area and cause seeding or drop metastases, which is seen in 1-2% of all OG cases.1 This case report is the first spinal metastasis case of a low-grade intracranial mass in our series so far.
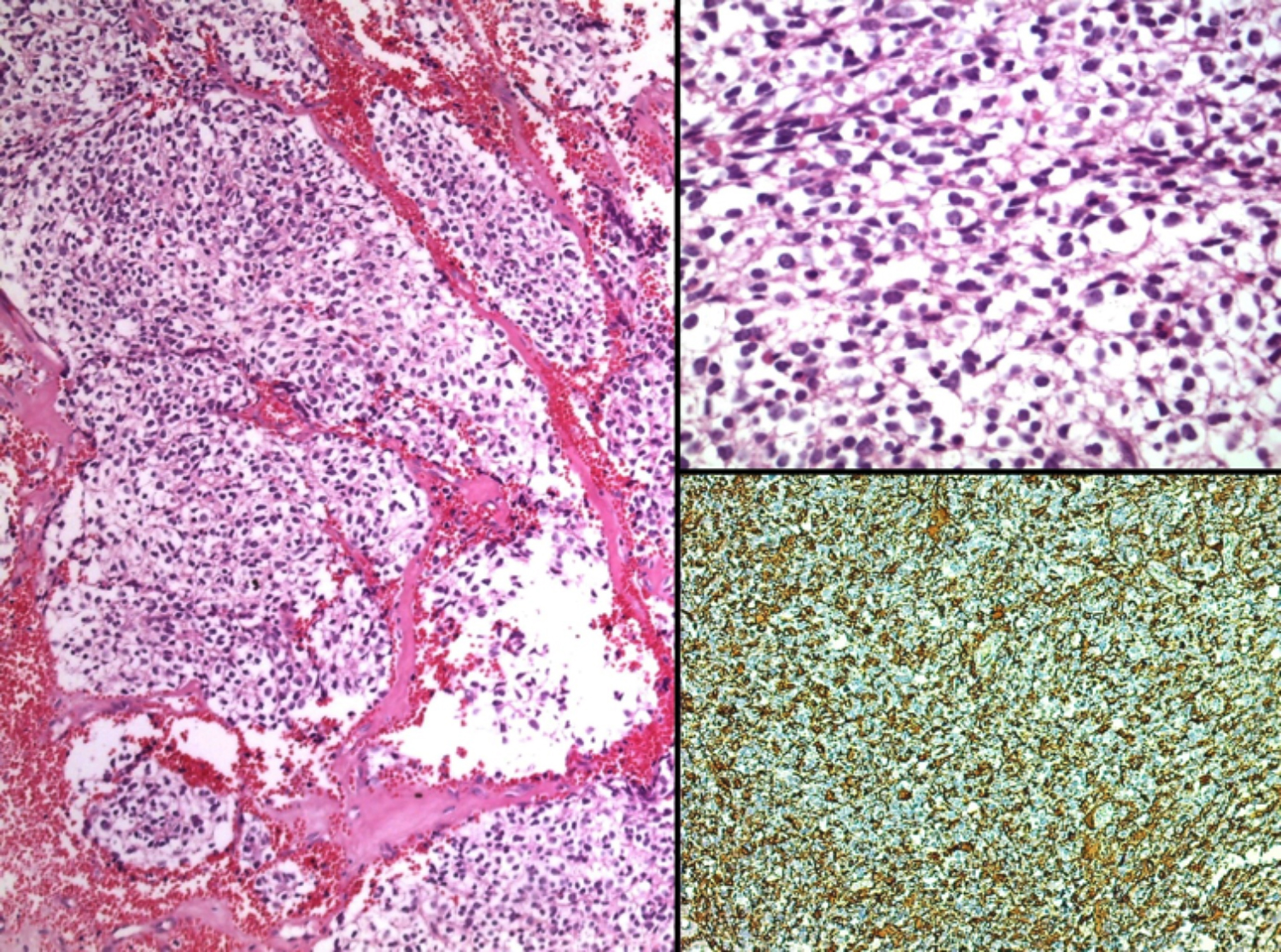 Figure 2: Oligodendroglioma showing monotonous tumour cells with round nuclei and perinuclear halo (left). Higher magnification showing characteristic tumour cells with perinuclear halo resulting in "fried egg" appearance (top right). Focal GFAP immunoreactivity (lower right).
Figure 2: Oligodendroglioma showing monotonous tumour cells with round nuclei and perinuclear halo (left). Higher magnification showing characteristic tumour cells with perinuclear halo resulting in "fried egg" appearance (top right). Focal GFAP immunoreactivity (lower right).
Despite the fact that seeding or drop metastases are the main cause of spinal metastases, there is no clear information on how they actually occur. Concerning this controversial issue, it was suggested that blood or lymphatic pathways may be involved.6 Cases where the interval between the first diagnosis and spinal metastasis was as long as 3 months to 6 years have been reported.7 In all reported cases, it has been observed that spinal cord metastases have occurred following cranial tumour surgery. In this case, similar to previous cases, spinal metastasis was detected 27 months after the cranial surgery.
Unlike low-grade OGs, anaplastic OGs are known to cause both intramedullary and extramedullary metastases. In low-grade OGs, it was observed that metastasis occurred when the tumour advanced in terms of its histopathological grade. In the literature, in the first OG case associated with seeding metastasis, it was observed that the histopathological grade of the lesion showed the transformation from low-grade OG to anaplastic OG 14 years after the first diagnosis.8 On the other hand, in the present case, symptomatic seeding metastasis occurred without malignant transformation. Velz et al. incidentally detected intradural extramedullary metastasis during another tumour screening in a patient diagnosed with anaplastic OG nine years after the first diagnosis of the lesion.6 Verma et al. found spinal cord (intramedullary) and multiple leptomeningeal metastases approximately six years after the diagnosis of low-grade OG.9 Their 37-year-old patient did not agree to surgical treatment for their metastatic lesions in the thoracolumbar region although she had pain, numbing, and weakness in her left leg, and her cerebrospinal fluid sampling result came out as negative. However, after radiotherapy, the patient’s complaints of pain decreased, and lesions improved, though partially. Although, the present case had a similar localisation, in contrast to the aforementioned patient without a pathological diagnosis, our 52-year-old patient with the severe neurological deficit was taken into surgery, and he received the diagnosis of low-grade OG. Nevertheless, after the diagnosis of spinal metastasis, the prognosis of our patient worsened. Spinal metastases are often asymptomatic, and in many cases, patients die before their metastases are detected. Becker et al. operated on a 16-month-old patient with a diagnosis of OG and performed V-P shunting. The patient died two months after the operation, and his postmortem examination revealed widespread metastases in the peritoneum.10 The decision made for surgical excision is important after the diagnosis of spinal metastases because with surgery, tumour cells can spread by gravity or by blood vessels, and consequently, spinal metastases may spread further.7 Additionally, the patient’s age, general condition, and presence of neurological deficit should be considered when deciding on surgery. Combined therapy (chemoradiotherapy) is the first and only treatment option in patients with diffuse metastases.8 In the present case, decompression surgery was performed for thoracic intradural-extramedullary compression, but the lesion that turned out to be WHO Grade 2 OG progressed towards the cervical region and the brain stem, and it was observed that the clinical status of the patient worsened further. As the patient had frequent seizures, and his clinical status continually deteriorated, he was not able to complete his radiotherapy treatment for spinal metastasis.
In conclusion, spinal seeding metastases may rarely occur in primary intracranial low-grade OGs. In addition to anaplastic OG types, it should be kept in mind that spinal metastases can be seen in OG without malignant transformation as in this case.
PATIENT’S CONSENT:
Informed consent has been obtained from the patient’s family to publish the case.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
EK: Concept, manuscript writing, data collection and data analysis.
HAA: Data analysis and manuscript review.
EK, MK, BB: Drafting the work or revising it critically for important intellectual content.
KS: Data collection.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Engelhard HH, Stelea A, Mundt A. Oligodendroglioma and anaplastic oligodendroglioma: Clinical features, treatment, and prognosis. Surgical Neurology 2003; 60(5):443-56. doi: 10.1016/S0090-3019(03)00167-8.
- Tork CA, Atkinson C. Oligodendroglioma. Stat Pearls 2020. www.ncbi.nlm.nih.gov/books/NBK559184.
- Ng HK, Sun DT, Poon WS. Anaplastic oligodendroglioma with drop metastasis to the spinal cord. Clinical Neurol Neurosurg 2002; 104(4):383-86. doi: 10.1016/s0303- 8467(02)00011-2.
- Morshed RA, Lau D, Sun PP, Ostling LR. Spinal drop metastasis from a benign fourth ventricular choroid plexus papilloma in a pediatric patient: Case report. J Neurosurg Pediatrics 2017; 20(5): 471-79. doi: 10.3171/2017.5. PEDS17130.
- Singh VK, Singh S, Bhupalam L. Anaplastic oligoden-droglioma metastasizing to the bone marrow: A unique case report and literature review. Int J Neurosci 2019; 129(7): 722-8. doi: 10.1080/00207454.2018.1557165.
- Velz J, Bellut D, Krayenbühl N, Winklhofer S, Rushing E, Frauenknecht K. 69-year-old male with an intradural, extramedullary mass at T12-L1. Brain Pathology 2019; 29(5):693-94. doi: 10.1111/bpa.12765.
- Pinar AO, Ilkay I, Hakan O, Figen S, Osman EO. Unusual massive spinal metastasis of an intracranial oligodendroglioma. Unusual massive spinal metastasis of an intracranial oligodendroglioma. Turkish Neuro Surg 2008; 18(3):276-80.
- Carlsen JG, Tietze A, Lassen YA, Rosendal F. Paraplegia due to drop metastases from anaplastic oligodendro-glioma. Br J Neurosurg 2012; 26(1):94-5. doi: 10.3109/ 02688697.2011.578767.
- Verma N, Nolan C, Hirano M, Young RJ. Intramedullary spinal cord and leptomeningeal metastases from intracranial low-grade oligodendroglioma. Clin Imag 2014; 38(4):505-07. doi: 10.1016/j.clinimag.2014.02.015.
- Becker H, Walter GF, Tritthart H, Oberbauer RW. Extraneural metastasis of an oligodendroglioma in ventriculo-peritoneal shunt. Onkologie 1978; 1(5):216-20. doi: 10.1159/000213955.