Primary Hepatic Sarcomatoid Carcinoma Presenting with Dysphagia: A Rare Presentation
By Khadim Hussain Samejo, Rajesh Mandhwani, Zain Majid, Muhammed Mubarak, Nasir Hassan LuckAffiliations
doi: 10.29271/jcpsp.2022.08.S159ABSTRACT
A 50-year male presented with vomiting and dysphagia for 2 weeks. Laboratory workup showed a positive serology for hepatitis C and normal serum α-fetoprotein (AFP) levels. CT abdomen revealed a large lesion in the right lobe of the liver extending upto the lower esophagus causing significant luminal narrowing and dysphagia. The enhancement pattern on the CT scan was not consistent with hepatocellular carcinoma. Liver lesion biopsy showed an infiltrating spindle cell lesion exhibiting fascicles of spindle cells with moderately hyperchromatic nuclei and perinuclear vacuolization. Mitotic count was 2-3/10 HPFs. Immunohistochemical markers were positive for CK AE1/AE3 and vimentin. Thus, a diagnosis of sarcomatoid carcinoma was made on the basis of morphological and immunohistochemical features. Due to unresectable disease and poor functional status, palliative care was opted for.
Key Words: Dysphagia, Vomiting, Liver biopsy, Sarcomatoid carcinoma.
INTRODUCTION
Sarcomatoid carcinoma (SC) is a rare primary malignant tumour of the liver. A study from Japan showed that during 1 year only 9 patients were found to have primary liver sarcomas in comparison to >19,000 and >900 cases of hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC), respectively.1
There is also a variant of HCC, called sarcomatoid HCC. Such sarcomatoid differentiation is often seen in those cases which have undergone locoregional therapy for HCC. Mostly spindle cells exhibit positivity for vimentin or desmin while showing an absence of albumin and α-fetoprotein (AFP) staining on immunohistochemistry (IHC). Some may also stain positive with cytokeratin (CK) marker.2
The sarcomatoid tumour of the liver consists of a mixture of epithelial (carcinomatous) and sarcomatous elements without the usual features of HCC or ICC. Most of the cases reported in the liver are usually seen in conjunction with HCC and ICC.2,3
We report a case of a 50-year man who presented with dysphagia. CT abdomen which revealed a large heterogeneous mass lesion in the liver, biopsy of that showed SC.
CASE REPORT
A 50-year male was admitted with the complaints of dysphagia and vomiting for 2 weeks. There was no history of fever, abdominal pain, jaundice, or other symptoms. The physical examination was unremarkable. He had poor functional status (ECOG 3). The serological marker for hepatitis C virus (HCV) was positive while HCV RNA by PCR (Abbot Realtime HCV) was not detected. His serum AFP (3.3 ng/ml; Normal: <8 ng/ml) was normal. CT scan abdomen showed ring-enhancing lesion of about 10.9 ×10.1 cm with central necrosis in the right lobe of the liver (Figure 1A) extending up to the sub-diaphragmatic region. Arterial enhancement and venous washout were not noted. The mass was found to be compressing and displacing inferior vena cava, right and left atria, pulmonary veins, and esophagus. More than 90% circumference of the thoracic aorta was encased by the tumour. Multiple large lymph nodal masses were noted in the chest and abdomen, the largest being 3.3 cm in maximum diameter (Figure 1B).
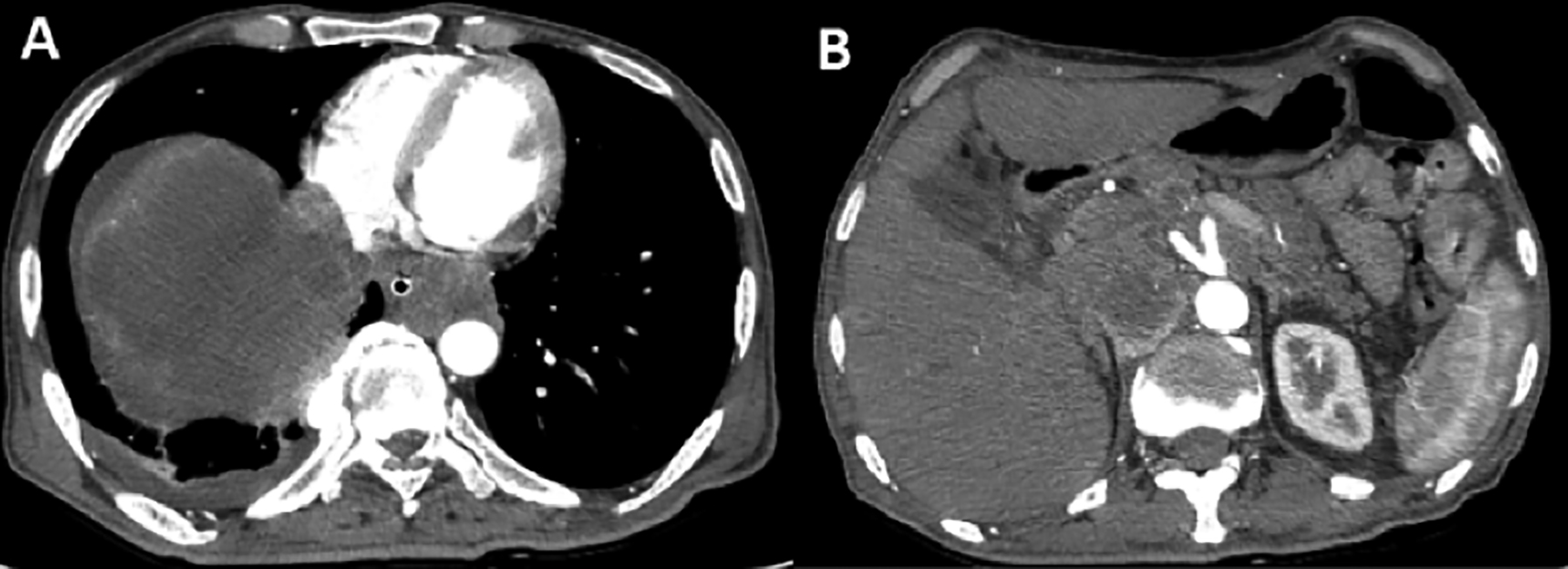 Figure 1: CT scan abdomen: A: A large heterogeneous mass lesion involving segment VII and VIII of the liver with central necrosis and peripheral rim enhancement. B. A large lymph nodal mass is shown to the left of abdominal aorta.
Figure 1: CT scan abdomen: A: A large heterogeneous mass lesion involving segment VII and VIII of the liver with central necrosis and peripheral rim enhancement. B. A large lymph nodal mass is shown to the left of abdominal aorta.
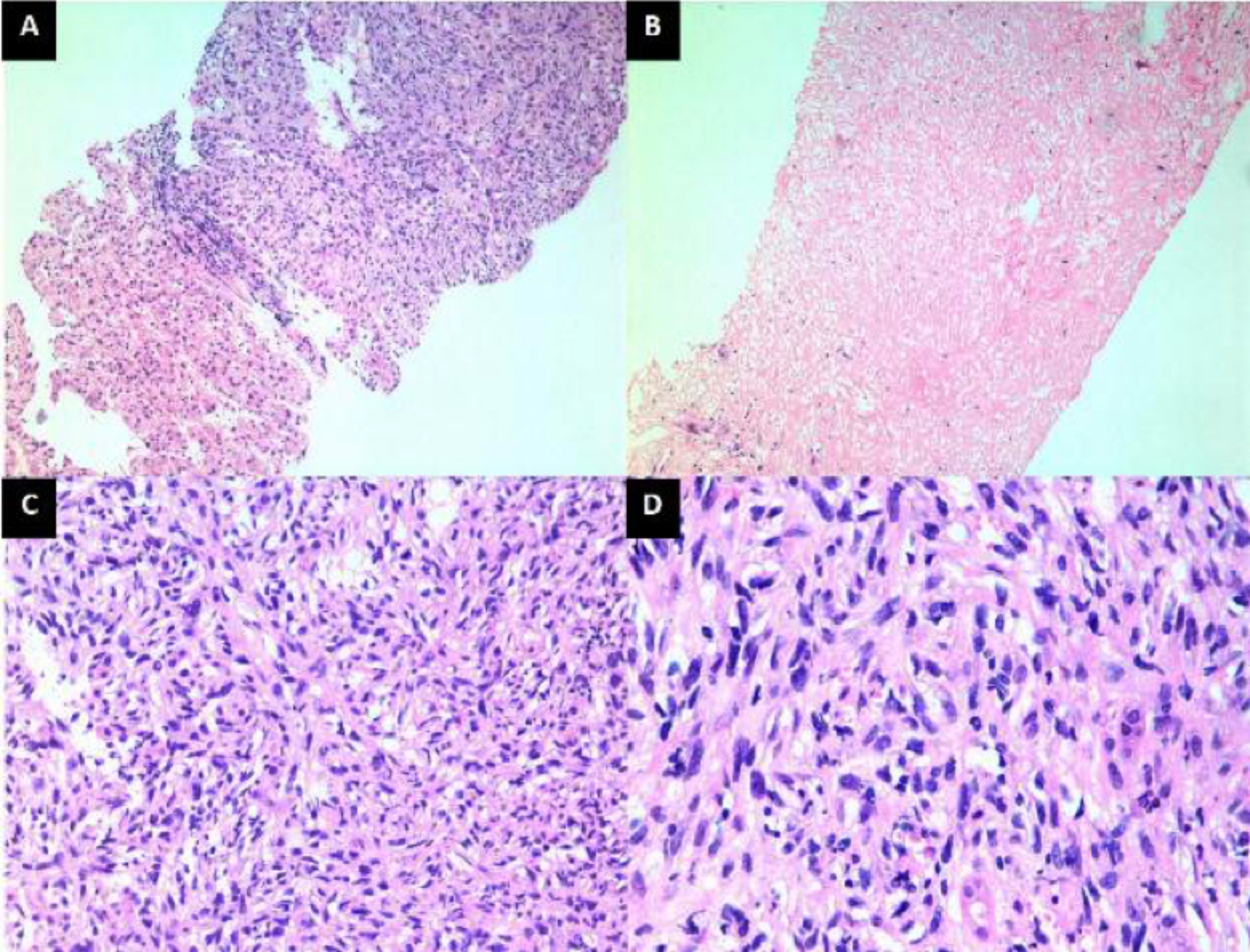 Figure 2: Liver biopsy findings on light microscopy. A. Low-power view showing both the neoplastic lesion and normal liver parenchyma (lower part of the picture). (H&E, ×100). B. Another area of biopsy showing complete tumour necrosis. (H&E, ×100). C. Medium-power view showing cellular composition of the tumour. Note that the tumour is composed of spindle cells arranged in irregularly irregular pattern. (H&E, ×200). D. High-power view showing spindle cells with hyperchromatic and mildly pleomorphic nuclei. (H&E, ×400).
Figure 2: Liver biopsy findings on light microscopy. A. Low-power view showing both the neoplastic lesion and normal liver parenchyma (lower part of the picture). (H&E, ×100). B. Another area of biopsy showing complete tumour necrosis. (H&E, ×100). C. Medium-power view showing cellular composition of the tumour. Note that the tumour is composed of spindle cells arranged in irregularly irregular pattern. (H&E, ×200). D. High-power view showing spindle cells with hyperchromatic and mildly pleomorphic nuclei. (H&E, ×400).
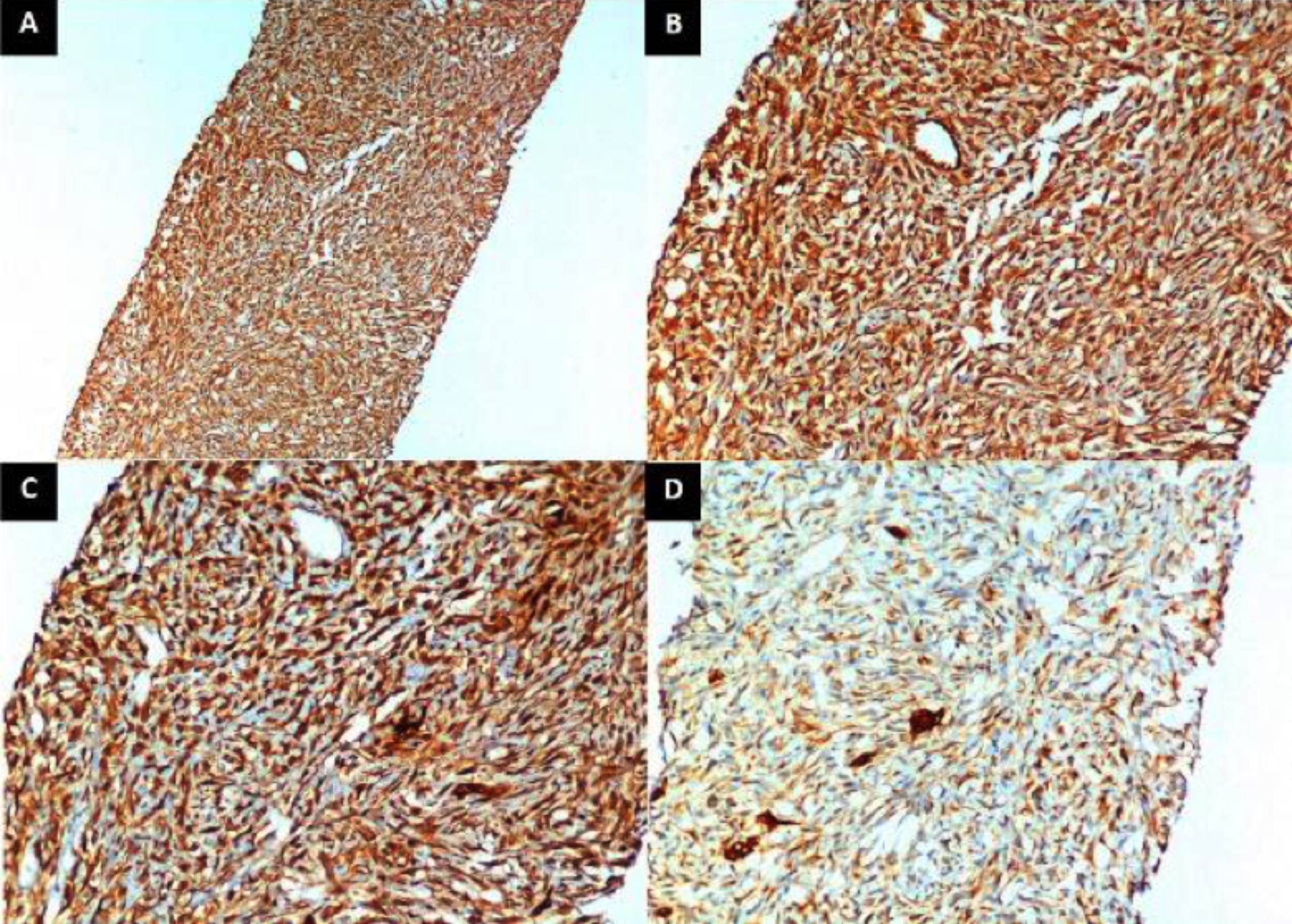 Figure 3: Immunohistochemistry (IHC) of the tumour. A. Low-power view showing diffuse positivity of tumour cells for vimentin (IHC for Vimentin, ×100). B. Medium-power view showing vimentin positivity of tumour cells. (IHC for Vimentin, ×200). C. Medium-power view showing diffuse positivity of cytokeratin (CK) AE1/AE3 in tumour cells. (IHC for CK AE1/AE3, ×200). D. Medium-power view showing diffuse weak positivity of CK 7 in tumour cells. A few scattered ductal structures show intense positivity. (IHC for CK 7, ×200).
Figure 3: Immunohistochemistry (IHC) of the tumour. A. Low-power view showing diffuse positivity of tumour cells for vimentin (IHC for Vimentin, ×100). B. Medium-power view showing vimentin positivity of tumour cells. (IHC for Vimentin, ×200). C. Medium-power view showing diffuse positivity of cytokeratin (CK) AE1/AE3 in tumour cells. (IHC for CK AE1/AE3, ×200). D. Medium-power view showing diffuse weak positivity of CK 7 in tumour cells. A few scattered ductal structures show intense positivity. (IHC for CK 7, ×200).
Esophagoduodenoscopy revealed an external compression of the esophagus at the level of 30-42 cm from incisors. Liver lesion biopsy was performed percutaneously which showed an infiltrating spindle cell lesion exhibiting fascicles of spindle cells with moderately hyperchromatic nuclei and perinuclear vacuolisation (Figure 2). Mitotic count was about 2-3/10 HPFs. Immunohistochemically, tumour was positive for CK 7, CK AE1/AE3, and vimentin; while it was negative for actin, caldesmon, S-100, CD 34, CD 117, DOG 1, CK 19, CK 20, and AFP (Figure 3). Thus, on the basis of above mentioned radiologic, histopathological, and IHC characteristics, he was diagnosed to have primary hepatic SC. But due to the unresectable nature of the disease and the poor functional status of the patient, palliative care was opted. In order to relieve dysphagia, an uncovered metallic esophageal stent of 23×150 mm was deployed (ultarflexTM esophageal NG-Boston scientific). He resumed his oral diet and was discharged home.
DISCUSSION
There are variants of HCC and ICC known as sarcomatoid HCC and sarcomatoid ICC with a mixture of epithelial (carcinomatous) and sarcomatous elements and typical features of HCC/ICC, in which sarcomatoid component predominates. Such sarcomatoid differentiation is often seen in those cases which have undergone locoregional therapy for such lesions like transarterial chemo-embolization (TACE), percutaneous ethanol injection and/or radiofrequency ablation.2 But pure SC of liver consists of a mixture of epithelial (carcinomatous) and sarcomatous elements without usual features of HCC or ICC. This patient neither had typical features of HCC nor ICC on histopathological evaluation nor underlying cirrhosis of liver and history of locoregional therapy.
Presenting symptoms are different from patients with HCC like abdominal pain and fever.4 This patient presented differently in the form of dysphagia and vomiting. Cross-sectional imaging has a vital role in the diagnosis of hepatic sarcomatoid tumours. Imaging usually shows heterogeneous neoplastic lesions with central necrosis and peripheral enhancements.5 For definitive diagnosis, histopathology and IHC is very important, showing both mesenchymal and epithelial components.2 CK, vimentin, and EMA are IHC stains used for its diagnosis, with CK8 being the most critical one used for establishing a diagnosis.6
Surgical excision of the tumour is the mainstay of treatment, while the effects of radiotherapy or chemotherapy are yet to be explored.7 The prognosis of this tumour is dismal because of its highly malignant potential, invasiveness, low resectability rates and more recurrence rates.8-10 In this case, tumour was fairly advanced with poor functional status, which deemed the tumour as unresectable and palliative care was instituted, similar to what was reported in the case report of Patel et al.9
In conclusion, primary hepatic SC is very aggressive and has an invasive biologic behaviour. Its accurate diagnosis is based upon histological and IHC analysis. The main treatment option is surgical excision of the tumour if identified early and the effects of radiotherapy or chemotherapy are not clear. This tumour carries a dismal prognosis because of its biological behaviour.
PATIENT’S CONSENT:
Informed written consent was obtained from the patient.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
KHS, RH: Managed the patient.
ZM: Wrote the manuscript.
MM: Histopath image.
NHL: Checked the revised version critically.
REFERENCES
- Nirei K, Matsuoka S, Moriyama M, Nakamura H, Maebayashi T, Takayama T, et al. Rare sarcomatoid liver carcinoma composed of atypical spindle cells without features of either HCC or ICC: A case report. Int J Clin Exp Med 2016; 9(10):20308-13.
- Giunchi F, Vasuri F, Baldin P, Rosini F, Corti B, D’Errico-Grigioni A. Primary liver sarcomatous carcinoma: Report of two cases and review of the literature. Pathol Res Pract 2013; 209(4):249-54. doi: 10.1016/j.prp.2013.01.005.
- Leng Q, Xiang XI, Tang Y, Yang Y, Qiu LI. Primary hepatic sarcomatoid carcinoma: A case report. ExpTher Med 2015; 10(3):1145-8. doi: 10.3892/etm.2015.2599.
- Eriguchi N, Aoyagi S, Hara M, Okuda K, Fukuda S, Tamae T, et al. Malignant sarcomatoid tumor of the liver: Report of a case. Surg Today 2001; 31(2):170-3. doi: 10.1007/s00 5950170205.
- Koo HR, Park MS, Kim MJ, Lim JS, Yu JS, Jin H, et al. Radiological and clinical features of sarcomatoid hepatocellular carcinoma in 11 cases. J Comput Assist Tomogr 2008; 32(5):745-9. doi: 10.1097/RCT.0b013 e3181591ccd.
- Haratake J, Horie A. An immunohistochemical study of sarcomatoid liver carcinomas. Cancer 1991; 68(1):93-7. doi: 10.1002/1097-0142(19910701)68:1<93:aid-cncr282 0680119>3.0.co;2-g.
- Maeda T, Adachi E, Kajiyama K, Takenaka K, Sugimachi K, Tsuneyoshi M. Spindle cell hepatocellular carcinoma. A clinicopathologic and immunohistochemical analysis of 15 cases. Cancer 1996; 77(1):51-7. doi: 10.1002/(SICI)1097- 0142(19960101)77:1<51::AID-CNCR10>3.0.CO;2-7.
- En Lin M, CH Huang. Recurrent hepatic sarcomatoid carcinoma cured by doxorubicin and ifosfamide chemotherapy. J Cancer Res Pract 2015; 2(3):241-7.
- Patel KB, Hawarny Rita H, Elizabeth S. Rare sarcomatoid carcinoma of the liver in a patient with no history of hepatocellular carcinoma: A case report. Marshall J Med 2019; 5(2), Article 3. doi: 10.33470/2379-9536.1215.
- Yu Y, Zhong Y, Wang J, Wu D. Sarcomatoid hepatocellular carcinoma (SHC): A case report. World J Surg Oncol 2017; 15(1):219. Published 2017 Dec 12. doi:10.1186/s12957- 017-1286-1.