Predictive Factors of Success in Sleeve Gastrectomy: One-Year Follow-up and the Significance of HALP Score
By Ferhat Cay, Ali DuranAffiliations
doi: 10.29271/jcpsp.2021.12.1406ABSTRACT
Objective: To investigate whether HALP score [hemoglobin, albumin, lymphocyte (LY), platelet] can predict weight loss in patients undergoing sleeve gastrectomy.
Study Design: A cohort study.
Place and Duration of Study: Department of General Surgery, Balıkesir University, Turkey from July 2019 to July 2020.
Methodology: One-year data of 64 patients, who underwent sleeve gastrectomy due to obesity were included for the analysis. The cut-off value of HALP score was calculated to be 41.2971. Those below the cut-off value were classified into Group 1 (low HALP score) and those above it were classified into Group 2 (high HALP score). Preoperative and postoperative body mass index (BMI), hemotologic parameters, platelet/LY ratios (PLR), and neutrophil/LY ratios (NLR), were compared. According to the percentage of excess BMI loss, patients were divided into 3 groups: Group A (≤40%), Group B (40%–60%), Group C (≥60%).
Results: The decrease in postoperative BMI was significantly high in Group 2 (p<0.001). There was a significant difference between the groups in terms of percentage change of hemoglobin (p=0.012). The increase in postoperative LY value and decrease in postoperative PLR value were significantly high in Group 1 (p=0.019, p=0.003). Furthermore, patient distribution was significantly different among groups A, B, and C (p <0.001). Comparison of groups showed a significant difference between groups A and B and between groups A and C, but not between groups B and C (p=0.006, p<0.001, and p=0.192, respectively).
Conclusion: In patients with high HALP scores, the rate of weight loss was higher, and most of their laboratory parameters were improved compared to those patients with low HALP scores.
Key Words: Body mass index, Sleeve gastrectomy, obesity, Weight loss, HAIP score.
INTRODUCTION
Obesity is closely associated with an individual’s health and may lead to serious problems. It has been classified as the sixth leading health risk factor, and constitutes a major part of the overall global burden of disease.1 Surgical treatment is accepted as a quick and effective method for individuals with excessive adipose tissue.2 In recent years, there has been an increase in bariatric surgery due to the increasing prevalence of obesity. Sleeve gastrectomy has been the most frequently used bariatric surgery method worldwide.3 It is preferred because it is relatively safe, has a short operation time, and produces effective results.4 However, markers predicting how effective, it is in which patient remain to be fully elucidated.
Studies demonstrate that overweightness and obesity induce inflammatory responses. Food consumption acutely induces inflammatory responses, and overnutrition is thought to yield the initial stimulus for the induction of inflammation. The stimulus originates from the tissues, such as adipose, liver and muscle tissues, that are involved in metabolism, and inflammatory response is triggered in response to this stimulus.5,6 All hematological parameters, including white blood cell (WBC) count [neutrophil (NE), lymphocyte (LY) and monocyte counts], platelet (PLT) count, and hemoglobin (HGB), albumin (ALB), C-reactive protein and fibrinogen levels, which are mostly observed to change during the inflammatory process, are reliable indicators of postoperative prognosis.7,8 It is known that combinations of these parameters, such as NE/LY ratio (NLR), PLT/LY ratio (PLR) and LY/monocyte ratio, are better predictors of prognosis. Recently, HGB, ALB, LY and PLT (HALP) score, which are used as prognostic markers, especially in colorectal, gastric and urinary system malignancies, have been developed. HALP score is thought to be an easily calculable score that demonstrates systemic inflammation and nutritional status and used as a prognostic factor in many cases of malignancies.9,10 However, to the best of authors’ knowledge, there is no study in the literature on the relationship between HALP score and obesity.
In this study, a one-year follow-up of 64 patients, who underwent sleeve gastrectomy due to morbid obesity, was conducted and the effectiveness of HALP score, as a predictive factor of weight loss, was investigated.
METHODOLOGY
The study included 64 patients, who underwent sleeve gastrectomy due to morbid obesity in the General Surgery Clinic, Faculty of Medicine, Balikesir University, Turkey between July 2019 and July 2020 were included. The study was approved by the University’s Non-Invasive Clinical Research Ethics Committee (approval number: 2021/188, date: 08.09.2021). The one-year follow-up results of the patients were analysed prospectively from the date of surgery, and a database was generated. A retrospective analysis was performed using this database. HALP scores of the patients were calculated using the following formula: HGB (g/L) × ALB (g/L) × LY (/L)/PLT (/L). Receiver operating characteristic (ROC) curve analysis was performed to evaluate the effectiveness of HALP score in differentiating successful and unsuccessful groups in terms of weight loss, and determine the cut-off value. For determining the cut-off value, surgery was considered successful in patients whose percentage of excess BMI loss (% EBMIL) was >40% at the end of one year.11 Using this cut-off value, the patients were divided into two groups — Group 1 (low HALP score) and Group 2 (high HALP score). Demographic characteristics and body mass index (BMI) of the patients were classified according to Group 1 and Group 2, and statistical analyses were performed. Values of WBC, HGB, PLT, LY, monocyte, NE, glucose, PLR, NLR, alanine aminotransferase (ALT), aspartate aminotransferase (AST), ALB and HALP scores were classified according to Group 1 and Group 2, and preoperative and postoperative results were analysed. According to % EBMIL, patients were divided into three groups — Group A (≤40% of EBMIL), Group B (40%–60% of EBMIL) and Group C (≥ 60% of EBMIL). % EBMIL was determined using the following formula: % EBMIL = Baseline BMI − Final BMI/Baseline BMI − Ideal BMI × 100.11,12 Patients with low and high HALP scores in these groups were determined and statistical analyses were performed.
Variables were tested for the presence of a normal distribution, using the Shapiro-Wilk test. For the comparison of two independent groups, independent samples t-test was used, if the assumption of normality was met; and mean and standard deviation (SD) were presented for descriptive variables. If the normality assumption was not met, then Mann-Whitney U-test was used, and median (minimum–maximum) values were presented for descriptive variables. For the comparison of two dependent groups, paired sample t-test was used, if the assumption of normality was met, and mean and SD were presented for descriptive variables. If the normality assumption was not met, Wilcoxon test was used, and median (minimum–maximum) values were presented for descriptive variables. Pearson’s Chi-square, Fisher’s Exact and Fisher–Freeman–Halton tests were used to compare categorical variables between groups, and categorical variables were presented as n (%) values. ROC curve analysis was used to evaluate the effectiveness of HALP score in differentiating successful and unsuccessful groups in terms of weight loss, and the cut-off value was determined according to the Youden’s J index criterion. Percentage change values for the variables were calculated using the following formula: postoperative value − preoperative value/preoperative value. In all statistical tests, α-value was set at <0.05, and two-tailed hypothesis test was used. Statistical analyses were performed using IBM SPSS Statistics version 23.0 and MedCalc 12.5.0.0 package programmes.
RESULTS
Sixty-four patients, who underwent sleeve gastrectomy due to obesity, were included in the study, to evaluate HALP score’s effectiveness in differentiating successful and unsuccessful groups. Those who lost more than 40% of excess BMI at the end of one year were considered surgically successful. In terms of weight loss and to determine the cut-off value, ROC curve analysis was performed. Accordingly, area under the curve was calculated to be 0.973 (p <0.001), and the cut-off value was calculated to be 41.2971. Above the determined cut-off value, the sensitivity of HALP score in predicting the success of sleeve gastrectomy was 88% (75.7% – 95.5%) and the specificity was 100% (76.8% – 100.0%). Those with HALP scores below and above 41.2971 were classified into Group 1 (low HALP scores) and Group 2 (high HALP scores), respectively.
Group 1 consisted of six males (31.6%) and 13 females (68.4%), and Group 2 consisted of 18 males (40%) and 27 females (60%) (p=0.525). The mean age of the patients was 36.21 ± 14.32 years in Group 1, and 34.44 ± 10.44 years in Group 2 (p=0.631). There was no significant difference between the two groups in terms of age, gender and preoperative BMI. While the preoperative PLT, PLR, NLR values were significantly low in Group 2, the LY value was significantly high (Table I).
Table I: Comparison between low- and high-HALP score groups in terms of patient demographics and preoperative characteristics.
|
Variables |
Low-HALP score group |
High-HALP score group |
p-value |
|
|
Age (years)* |
36.21±14.32 |
34.44±10.44 |
0.631 |
|
|
Gender& |
Male |
6 (31.6) |
18 (40.0) |
0.525 |
|
Female |
13 (68.4) |
27 (60.0) |
||
|
BMI # |
47.20 (40.30-50.20) |
46.30 (40.00-50.00) |
0.453 |
|
|
HGB* |
12.98±1.33 |
13.50±1.34 |
0.168 |
|
|
PLT# |
333.00 (245-442) |
289.00 (125-638) |
0.015 |
|
|
WBC# |
9.30 (6.30-16.80) |
8.70 (6.10-13.10) |
0.394 |
|
|
MPV# |
8.10 (6.70-10.50) |
8.10 (6.40-94.00) |
0.930 |
|
|
NE## |
5.50 (3.70-12.00) |
5.30 (3.90-11.80) |
0.522 |
|
|
LY#* |
2.17±0.44 |
2.59±0.78 |
0.008 |
|
|
MO# |
0.60 (0.20-0.90) |
0.50 (0.30-0.90) |
0.823 |
|
|
Glucose# |
106.00 (81-138) |
100.5 (08.90-227.00) |
0.381 |
|
|
PLR# |
160.95 (108.75-214.67) |
119.67 (62.43-278.57) |
0.001 |
|
|
NLR# |
2.78 (1.38-4.62) |
2.13 (1.15-6.29) |
0.011 |
|
|
AST# |
19.00 (12.00-36.00) |
20.00 (10.00-94.00) |
0.541 |
|
|
ALT# |
21.00 (10.00-54.00) |
29.00 (4.40-191.00) |
0.219 |
|
|
ALB# |
4.30 (3.80-4.80) |
4.30 (3.30-4.70) |
0.310 |
|
|
HALP# |
34.81 (25.26-39.96) |
54.93 (41.30-91.62) |
<0.001 |
|
|
Data are presented as *mean ± standard deviation, #median (minimum–maximum), or &n (%). |
||||
Table II: Comparison between low- and high-HALP score groups in terms of preoperative and postoperative laboratory values.
|
Variables |
Low-HALP score group |
High-HALP score group |
||||
|
Preoperative |
Postoperative |
p-value |
preoperative |
Postoperative |
p-value |
|
|
BMI |
45.82±3.29 |
36.51±3.64 |
<0.001* |
46.30 (40.00-50.00) |
29.00 (25.60-39.00) |
<0.001# |
|
HGB |
12.98±1.33 |
13.66±1.34 |
0.050* |
13.70 (10.30-15.80) |
13.70 (9.10-15.60) |
0.227# |
|
PLT |
338.00±52.92 |
274.79±66.07 |
<0.001* |
289.00 (125-638) |
271.00 (40.50-587.00) |
0.009# |
|
WBC |
9,30 (6.30-16.80) |
8.30 (4.70-15.10) |
0.011# |
8.70 (6.10-13.10) |
7.10 (4.20-11.90) |
0.001# |
|
MPV |
8.29±1.01 |
8.82±1.31 |
0.056* |
8.10 (6.40-94.00) |
8.50 (6.50-12.40) |
0.548# |
|
NE# |
5.50 (3.70-12.00) |
5.10 (2.60-10.30) |
0.033# |
5.30 (3.90-11.80) |
4.10 (1.80-8.30) |
0.001# |
|
LY# |
2.20 (1.50-3.20) |
2.40 (1.60-3.90) |
0.048# |
2.59±0.78 |
2.41±0.63 |
0.131* |
|
MO# |
0.58±0.18 |
0.53±0.21 |
0.180* |
0.50 (0.30-0.90) |
0.50 (0.20-0.90) |
0.042# |
|
Glucose |
107.58±16.20 |
88.21±9.00 |
<0.001* |
100.50 (8.90-227.00) |
87.00 (70.00-130.00) |
<0.001# |
|
PLR |
159.24±28.90 |
117.26±38.91 |
0.001* |
119.67 (62.43-278.57) |
106.25 (17.61-255.22) |
0.756# |
|
NLR |
2.87±0.86 |
2.16±0.67 |
0.007* |
2.13 (1.15-6.29) |
1.81 (0.81-4.88) |
0.022# |
|
AST |
19.00 (12.00-36.00) |
16.00 (9.00-39.00) |
0.372# |
20.00 (10.00-94.00) |
15.00 (9.00-196.00) |
0.026# |
|
ALT |
21.00 (10.00-54.00) |
14.00 (8.00-53.00) |
0.058# |
29.00 (4.40-191.00) |
11.00 (6.00-347.00) |
0.001# |
|
ALB |
4.30 (3.80-4.80) |
4.20 (2.60-4.70) |
0.038# |
4.30 (3.30-4.70) |
4.20 (2.60-4.80) |
0.482# |
|
Data are presented as mean ± standard deviation or median (minimum–maximum). *Paired samples t test and #Wilcoxon test was used. BMI: Body Mass Index; White Blood Cell: WBC; Hemoglobin: HG; Platelet: PLT; Lymphocyte: LY; Monocyte: MO; Neutrophil: NE; Platelet/Lymphocyte Ratio: PLR; Neutrophil / Lymphocyte Ratio: NLR; Alanine Aminotransferase: ALT; Aspartate Aminotransferase: AST; Albumin: ALB; HALP: Hemoglobin, Albumin, Lymphocyte and Platelet. |
||||||
Table III: Comparison between low- and high-HALP score groups in terms of percentage change in laboratory values.
|
Percentage |
Low-HALP |
High-HALP |
p-value |
|
BMI# |
-0.16(-0.37/-0.13) |
-0.35(-0.47/-0.17) |
<0.001 |
|
HGB# |
0.05(-0.18/0.30) |
-0.01(-0.15/0.31) |
0.012 |
|
PLT# |
-0.09(-0.51/0.00) |
-0.07(-0.90/0.57) |
0.101 |
|
WBC* |
-0.12±0.17 |
-0.13±0.24 |
0.763 |
|
MPV# |
0.04(-0.16/0.53) |
0.00(-0.90/0.26) |
0.160 |
|
NE# |
-0.21(-0.62/0.73) |
-0.25(-0.70/0.98) |
0.374 |
|
LY# |
0.19(-0.38/0.70) |
0.00(-0.56/1.14) |
0.019 |
|
MO* |
-0.08±0.25 |
-0.07±0.28 |
0.829 |
|
Glucose# |
-0.14(-0.43/-0.02) |
-0.16(-0.60/8.33) |
0.964 |
|
PLR# |
-0.28(-0.61/0.43) |
-0.03(-0.94/0.99) |
0.003 |
|
NLR# |
-0.31(-0.67/1.36) |
-0.20(-0.68/3.18) |
0.504 |
|
AST# |
-0.14(-0.56/1.17) |
-0.19(-0.67/1.60) |
0.558 |
|
ALT# |
-0.36(-0.76/0.76) |
-0.43(-0.88/1.67) |
0.214 |
|
ALB# |
-0.02(-0.38/0.09) |
0.00(-0.21/0.21) |
0.201 |
|
&Percentage change values are calculated using the following formula: [(postoperative value − preoperative value)/preoperative value]. |
|||
In Group 1, there was a significant decrease in BMI, PLT, WBC, NE, glucose, PLR, NLR and ALB values; whereas, there was a significant increase in LY value in the postoperative period compared to that in the preoperative period. Group 2 showed a significant decrease in BMI, PLT, WBC, NE, monocytes, glucose, NLR, AST and ALT values in the postoperative period compared to those in the preoperative period (Table II).
The amount of decrease in BMI in the postoperative period was found to be significantly higher in Group 2 than in Group 1 (p<0.001). While the median percentage change in HGB in Group 1 was 0.05 (minimum–maximum: −0.18–0.30), the median percentage change in Group 2 was −0.01 (minimum–maximum: −0.15–0.31); there was a significant difference between the two groups in terms of percentage change in HGB (p=0.012). The amount of increase in LY value in the postoperative period was found to be significantly higher in Group 1 than in Group 2 (p=0.019). The amount of decrease in PLR in the postoperative period was found to be significantly higher in Group 1 than in Group 2 (p=0.003) (Table III, Figure 1).
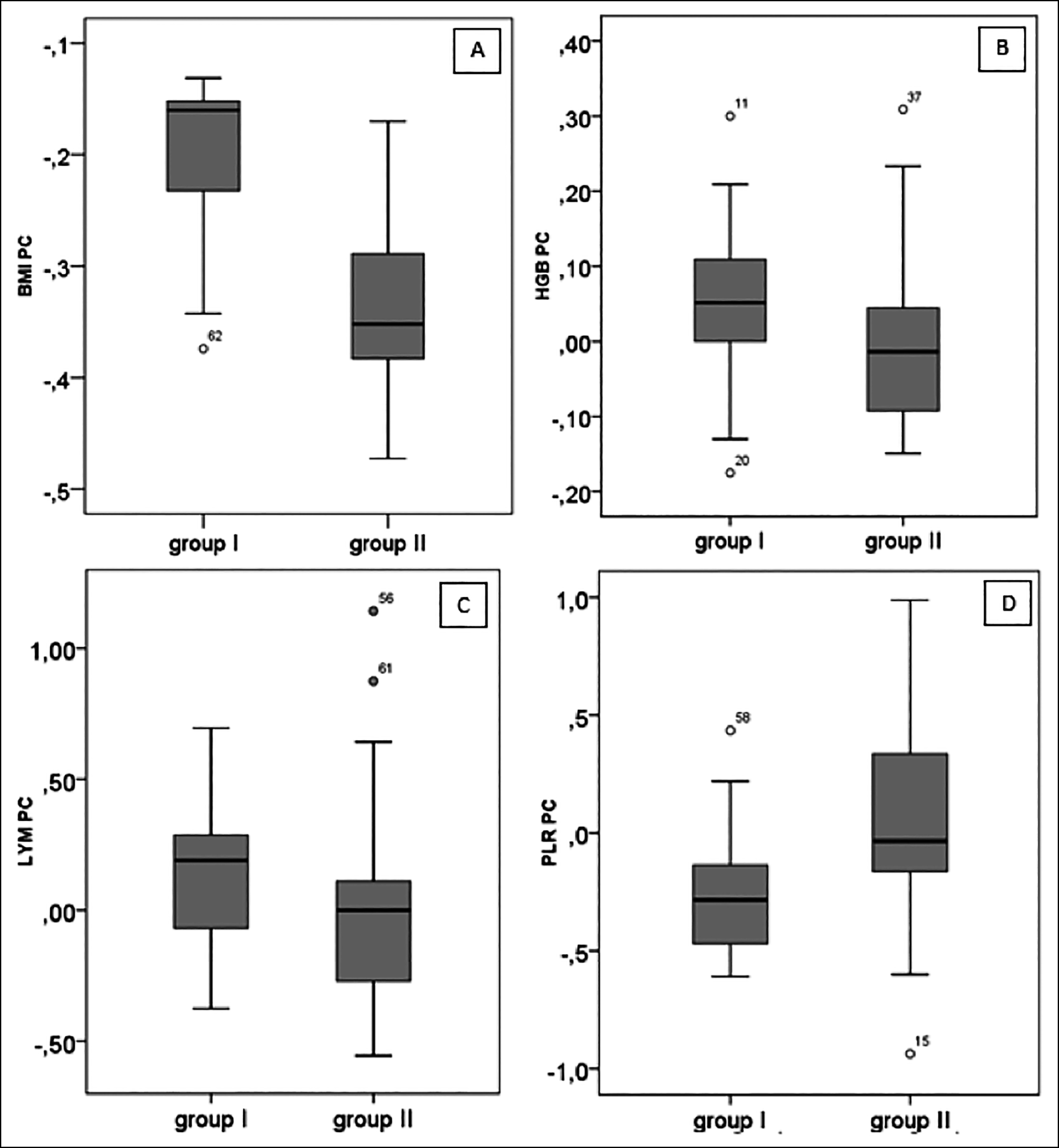 Figure I: Box-plot of percentage change in low- and high-HALP score groups: A) Percentage change in BMI, B) Percentage change in HGB, C) Percentage change in lymphocyte, and D) Percentage change in PLR (Group 1: low-HALP score group, Group 2: high HALP score group, PC: percentage change).
Figure I: Box-plot of percentage change in low- and high-HALP score groups: A) Percentage change in BMI, B) Percentage change in HGB, C) Percentage change in lymphocyte, and D) Percentage change in PLR (Group 1: low-HALP score group, Group 2: high HALP score group, PC: percentage change).
Patients with EBMIL of ≤40% were classified into Group A, 40%–60% were classified into Group B, and ≥60% were classified into Group C. The distribution of patients in these three groups showed a significant difference compared to that of patients in the high- and low-HALP score groups (p<0.001). When the groups were compared in pairs, there was a significant difference between Group A and B (p=0.006) and Group A and C (p<0.001); whereas, there was no significant difference between Group B and C (p=0.192).
DISCUSSION
Obesity is known to be associated with chronic inflammatory response. Therefore, it was thought that inflammatory parameters may have a prognostic value. HALP score, which is calculated using HGB, albumin, LY, and PLT values, has shown to have prognostic value in many types of malignancies and is closely associated with the inflammatory process.5,9 In this study, the authors investigated the relationship between HALP score and percentage of weight loss in individuals with obesity after sleeve gastrectomy.
The mean age of the patients included in the study was 36.21 ± 14.32 years in low-HALP score group and 34.44 ± 10.44 in high-HALP score group. While the present study revealed no statistically significant difference between the groups, HALP score in this study was quite low considering the average age when compared to that in the literature.9,10 This difference is due to the fact that in the literature, studies on HALP score include patients with advanced age, chronic diseases, or malignancies. Furthermore, there was no difference between the groups in terms of gender and preoperative BMI.
PLTs are involved in the release of proteins and metabolites that play an active role in processes such as sepsis, inflammation and tissue regeneration. PLTs increase tumour growth and angiogenesis by releasing many growth factors such as PLT-derived growth factor, transforming growth factor beta and vascular endothelial growth factor. These mechanisms have been associated with poor prognosis of cardiovascular diseases and several types of cancers. Similarly, PLR and NLR values are parameters that can be obtained via simple blood tests and could indicate poor prognosis in several types of cancers, ischemic diseases, chronic diseases such as coronary artery diseases, inflammatory conditions and nutritional disorders.13-16 In this study, preoperative PLT, PLR and NLR values were significantly low in Group 2; whereas, preoperative LY value was found to be significantly high (Table I).
On examination of the metabolic and hematological parameters, significant differences were found in pre-operative and postoperative BMI, PLT, WBC, NE, LY, glucose, PLR, NLR and ALB values in patients of Group 1. In this group, there was a significant decrease in BMI, PLT, WBC, NE, glucose, PLR, NLR and ALB values, but there was a significant increase in LY value in the postoperative period compared to that in the preoperative period. Group 2 showed a significant decrease in postoperative values of BMI, PLT, WBC, NE, monocytes, glucose, NLR, AST and ALT compared to their preoperative values (Table II). In studies available in the literature, hematological and metabolic parameters changed in a similar manner in the follow-ups conducted after sleeve gastrectomy, but there is no data on the HALP scores of patient groups.17-19 In this study, when metabolic and hematological parameters were compared between the groups, the amount of decrease in BMI in the postoperative period was found to be significantly higher in Group 2 than in Group 1 (p<0.001). The median percentage change in HGB in Group 1 was 0.05 (minimum–maximum: −0.18–0.30), and the median percentage change in Group 2 was −0.01 (minimum–maximum: −0.15–0.31). There was a 5% increase in median percentage change values of HGB in Group 1, whereas Group 2 showed a change of 1%. There was a significant difference between the two groups in terms of HGB percentage change (p=0.012). The amount of increase in LY value in the postoperative period was found to be significantly higher in Group 1 than in Group 2 (p=0.019). Although the amount of decrease in PLR value in the postoperative period was significantly higher in Group 1; than in Group 2, (p=0.003), there was no significant difference between the two groups in terms of PLT and NLR values (Table III, Figure 1).
Formulas for BMI change, percentage of total weight loss (%TWL), percentage of excess weight loss (% EWL), and % EBMIL or estimated body weight loss have been developed; the obtained values act as indicators of surgical success in bariatric surgeries. In this study, the success of surgery was determined using %EBMIL (% EBMIL = Baseline BMI − Final BMI/Baseline BMI − Ideal BMI × 100).12,20 In the literature, % EBMIL rate, which is an indicator of success in bariatric surgery, between 40%–60% is considered.11,21,22 Based on the values in the literature, patients with EBMIL ≤40% were classified into the first group, 40%–60% in the second group and ≥60% in the third group. A significant difference was found between Group 1 and Group 2 in terms of patient distribution in these three groups (p <0.001). When the groups were compared in pairs, there was a significant difference between groups A and B (p=0.006) and between groups A and C (p<0.001), but no significant difference was found between groups B and C (p=0.192).
The limited number of patients, short follow-up period, and retrospective analysis of the data are limitations in this study. But it may contribute to the literature, since it is the first study on this subject in the literature.
CONCLUSION
Patients with a high HALP score had a higher rate of weight loss than those with a low HALP score. In addition, patients with a high HALP score showed an improvement in most of the laboratory parameters.
ETHICAL APPROVAL:
Study was approved by the University’s Non-Invasive Clinical Research Ethics Committee (approval No. 2021/188, date: 08.09.2021).
PATIENTS’ CONSENT:
Since the study was a retrospective archive search, informed consent was not obtained from the patients.
CONFLICTC OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
Both authors contributed equally at all stages of the article.
REFERENCES
- Haslam DW, James WP. Obesity. Lancet 2005; 366: 1197-209.
- Shi X, Karmali S, Sharma AM, Birch DW. A review of laparoscopic sleeve gastrectomy for morbid obesity. Obesity Surg 2010; 20(8):1171-7. doi: 10.1007/ s11695- 010-0145-8.
- Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, et al. Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014. Obes Surg 2017; 27(9):2279-89. doi: 10.1007/s11695-017-2666-x.
- Rosenthal RJ, International Sleeve Gastrectomy Expert Panel, Diaz AA, Arvidsson D, Baker RS, Basso N, et al. International sleeve gastrectomy expert panel consensus statement: best practice guidelines based on experience of > 12,000 cases. Surg Obes Relat Dis 2012; 8(1):8-19. doi: 10.1016/j.soard.2011.10.019.
- Faloia E, Michetti G, de Robertis M, Luconi MP, Furlani G, Boscaro M. Inflammation as a link between obesity and metabolic syndrome. J Nutr Metab 2012. doi: 10.1155/ 2012/476380.
- Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell Metab 2012; 15(1):10-8. doi: 10.1016/j.cmet.2011.10.011.
- Jo JK, Jeong SJ, Hong SK, Byun SS, Lee SE, Oh JJ. The impact of preoperative anemia on oncologic outcome in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Int Urol Nephrol 2016; 48(4):489-94. doi: 10.1007/s11255-016-1219-x.
- Klinga G, Sherif A. A retrospective evaluation of preoperative anemia in patients undergoing radical cystectomy for muscle-invasive urothelial urinary bladder cancer, with or without neoadjuvant chemotherapy. Springer Plus 2016; 5(1):1167. doi: 10.1186/s40064- 016-2865-2.
- Xu SS, Li S, Xu HX, Li H, Wu CT, Wang WQ, et al. Haemoglobin, albumin, lymphocyte and platelet predicts postoperative survival in pancreatic cancer. World J Gastroenterol 2020; 26(8):828-38. doi: 10.3748/wjg.v26. i8.828.
- Guo Y, Shi D, Zhang J, Mao S, Wang L, Zhang W, et al. The hemoglobin, albumin, lymphocyte, and platelet (HALP) score is a novel significant prognostic factor for patients with metastatic prostate cancer undergoing cytoreductive radical prostatectomy. J Cancer 2019; 10(1):81-91. doi: 10.7150/jca.27210.
- Melton GB, Steele KE, Schweitzer MA, Lidor AO, Magnuson TH. Suboptimal weight loss after gastric bypass surgery: Correlation of demographics, comorbidities, and insurance status with outcomes. J Gastrointest Surg 2008; 12(2): 250-5. doi: 10.1007/s11605-007-0427-1.
- Hatoum IJ, Kaplan LM. Advantages of percent weight loss as a method of reporting weight loss after Roux‐en‐Y gastric bypass. Obesity (Silver Spring) 2013; 21(8):1519-25. doi: 10.1002/oby.20186.
- Lee JS, Kim NY, Na SH, Youn YH, Shin CS. Reference values of neutrophil–lymphocyte ratio, lymphocyte–monocyte ratio, platelet–lymphocyte ratio, and mean platelet volume in healthy adults in South Korea. Medicine 2018; 97(26): e11138. doi: 10.1097/MD.0000000000011138.
- Hudzik B, Szkodziński J, Lekston A, Gierlotka M, Poloński L, Gąsior M. Mean platelet volume-to-lymphocyte ratio: A novel marker of poor short- and long-term prognosis in patients with diabetes mellitus and acute myocardial infarction. J Diabetes Its Complications 2016; 30(6):1097-102. doi: 10.1016/j.jdiacomp.2016.04.010.
- Zhao Y, Si G, Zhu F, Hui J, Cai S, Huang C, et al. Prognostic role of platelet to lymphocyte ratio in hepatocellular carcinoma: A systematic review and meta-analysis. Oncotarget 2017; 8(14):22854-62. doi: 10.18632/ oncotarget.15281.
- Tsujino T, Komura K, Ichihashi A, Tsutsumi T, Matsunaga T, Yoshikawa Y, et al. The combination of preoperative platelet count and neutrophil lymphocyte ratio as a prognostic indicator in localised renal cell carcinoma. Oncotarget 2017; 8(66):110311-25.
- Aykota MR, Yilmaz S, Atabey M, Ozgen U, Simsek S. Effect of sleeve gastrectomy on the neutrophil-to-lymphocyte ratio, the platelet-to-lymphocyte ratio, platelet counts, and mean platelet volumes. Indian J Surg 2021; 83:261-7.
- Ertugrul I, Kuzu F. The impact of bariatric surgery on hematological inflammatory parameters. Ann Med Res 2019; 26:2250-4.
- Kyio NH, Turgut S, Ozkan T, Cetin G. Evolution of hemato-logical parameters during the first 2 years after laparoscopic sleeve gastrectomy: Results of a retrospective study. Obes Surg 2020; 30(7):2606-11. doi: 10.1007/ s11695-020-04528-x.
- Brethauer SA, Kim J, El Chaar M, Papasavas P, Eisenberg D, Rogers A, et al. Standardised outcomes reporting in metabolic and bariatric surgery. Obes Surg 2015; 11(3):489-506. doi: 10.1016/j.soard.2015.02.003.
- Chen CY, Lee CH, Lee HM, Yin WY, Chin WL, Lee MH, et al. Check point to get adequate weight loss within 6-months after laparoscopic sleeve gastrectomy for morbid obesity in Asian population. Sci Rep 2020; 10(1):12788. doi: 10.1038/s41598-020-69714-4.
- Zetu C, Popa S, Golli AL, Condurache A, Munteanu R. Long-term improvement of dyslipidaemia, hyperuricemia and metabolic syndrome in patients undergoing laparoscopic sleeve gastrectomy. Arch Endocrinol Metab 2021; 64(6):704-9. doi: 10.20945/2359-3997000000273.