Malignant Peripheral Nerve Sheath Tumor of the Cervix
By Tiantian Zhang, Zishu Wang, Rui Wang, Xiao Wu, Menglin Zhao, Fang SuAffiliations
doi: 10.29271/jcpsp.2022.04.24ABSTRACT
The occurrence of primary malignant peripheral neurilemmoma in the female cervix without neurofibromatosis type 1 is extremely rare. We, herein, report a case of a 46-year woman with malignant peripheral nerve sheath tumor (MPNST) involving the cervix. The patient was admitted to the hospital because of irregular vaginal bleeding. Pelvic CT suggested the presence of cervical mass, and the patient accepted radical hysterectomy. Following the surgery, MPNST was diagnosed by a combination of histology and immunohistochemistry. One year later, the patient developed recurrence and pulmonary metastasis and received sequentially epiubicin/ifosfamide, dacarbazine, and etoposide/cisplatin multi-line chemotherapy. The prognosis of MPNST is poor, which is related to its invasiveness, high recurrence and metastasis rates. The patient was treated with surgery, multi-line chemotherapy and targeted therapy, but the overall survival (OS) was 44 months. It is the first reported case of cervical MPNST receiving combined treatment.
Key Words: Malignant peripheral nerve sheath tumor, Cervix, Pulmonary metastasis, Hysterectomy, Chemotherapy.
INTRODUCTION
Malignant peripheral nerve sheath tumors (MPNSTs) are rare high-grade sarcomas arising from peripheral nerves or peripheral nerve sheath cells. The prevalence of MPNSTs in the general population is approximately 0.001%, accounting for 5-10% of all soft tissue sarcomas.1 These tumors usually affect the proximal extremities and the trunk; and are very rare in the pelvic viscera.2 These usually arise in adulthood, with most occurring around 20–50 years of age, and the incidence is equal in males and females.1 The diagnosis of MPNSTs depends mostly on the combination of histology and immunohistochemistry. There is no specific immunohistochemical marker for MPNSTs, but the neurological markers, S-100, CD 56, and PGP 9.5 are considered to be sensitive markers of peripheral nerve sheath tumors.3 Although S-100 protein has been known to be the best marker, but only 50-90% of MPNSTs show positivity with this marker.4
CASE REPORT
A 46-year woman presented to a local hospital because of irregular vaginal bleeding in January 2015. There was no evident abnormality in the examination. She denied a history of neurofibromatosis 1 (NF-1). She had two cesarean sections performed in 1999 and 2009, respectively.
The pelvic CT showed visible cervical lumps (Figure 1). The cervical biopsy showed a neurogenic malignant tumor, possibly MPNST. She underwent a radical hysterectomy, combined with bilateral adnexal resection and pelvic lymphadenectomy. The postoperative pathology showed malignant cervical spindle cell tumor (Figure 2A), consistent with MPNST. By immunohistochemistry, the cells were vimentin and S-100 positive (Figure 2B), and cytokeratin (CK), CD117, and CD34 were negative.
After surgery, five cycles of epirubicin combined with isocyclophosphamide and cisplatin, were administered. No recurrence or metastasis was found on the last imaging examination in June 2015. No abnormality was found during periodic review (Figure 3A).
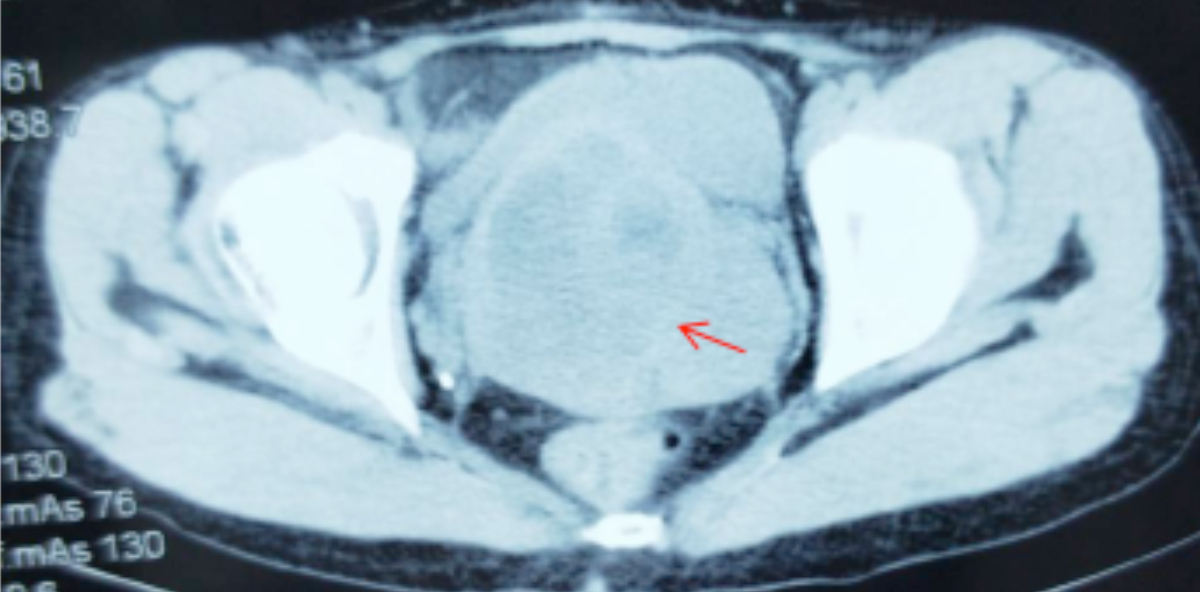 Figure 1: CT scan of the pelvis showing cervical masses.
Figure 1: CT scan of the pelvis showing cervical masses.
One year later, in January 2016, the patient was found to have a recto-vaginal fistula (Figure 3B), and multiple chest nodules were seen in the chest CT (Figure 4A), suggesting the possibility of lung metastasis. Following vaginal mass resection, the postoperative pathology showed recurrent MPNST. The patient was given gemcitabine, combined with docetaxel for four cycles of first-line chemotherapy. In June 2016, a review of pulmonary lesions’ progression was performed (Figure 4B). The second-line treatment was switched to anthracycline (AI, epiubicin combined with ifosfamide) chemotherapy for eight cycles. During repeated reviews, the lung and pelvic lesions remained stable till February 2017 (Figure 4C). Since the anthracycline had reached the maximum cumulative dose (1000 mg/m2), it was changed to dacarbazine alone to maintain chemotherapy. In October 2017, after two cycles of treatment, the condition was still stable (Figure 4D). After three cycles, in January 2018, the lung lesions on chest CT were reviewed (Figure 4E), showing progression of lesions, but no related symptoms were reported. A third-line treatment with EP (etoposide combined with cisplatin) was initiated. Cough, expectoration, and chest tightness occurred after one cycle of chemotherapy with the EP regimen. Chest B-ultrasound showed a large amount of pleural effusion. Thoracentesis yielded hemorrhagic pleural effusion. Chemotherapy was switched to gemcitabine plus docetaxel for one course of treatment. In July 2018, the chest and abdomen CT showed that the lung metastases increased further (Figure 4F), and nodular lesion appeared in front of the rectum. The combination of imaging studies indicated a progression of the disease and local recurrence. The multi-line chemotherapy was not effective, and the patient was subjected to targeted treatment with anlotinib (12mg, p.o. qd, q3w). The patient died two months after taking anlotinib. Unfortunately, no imaging examinations were performed during that period.
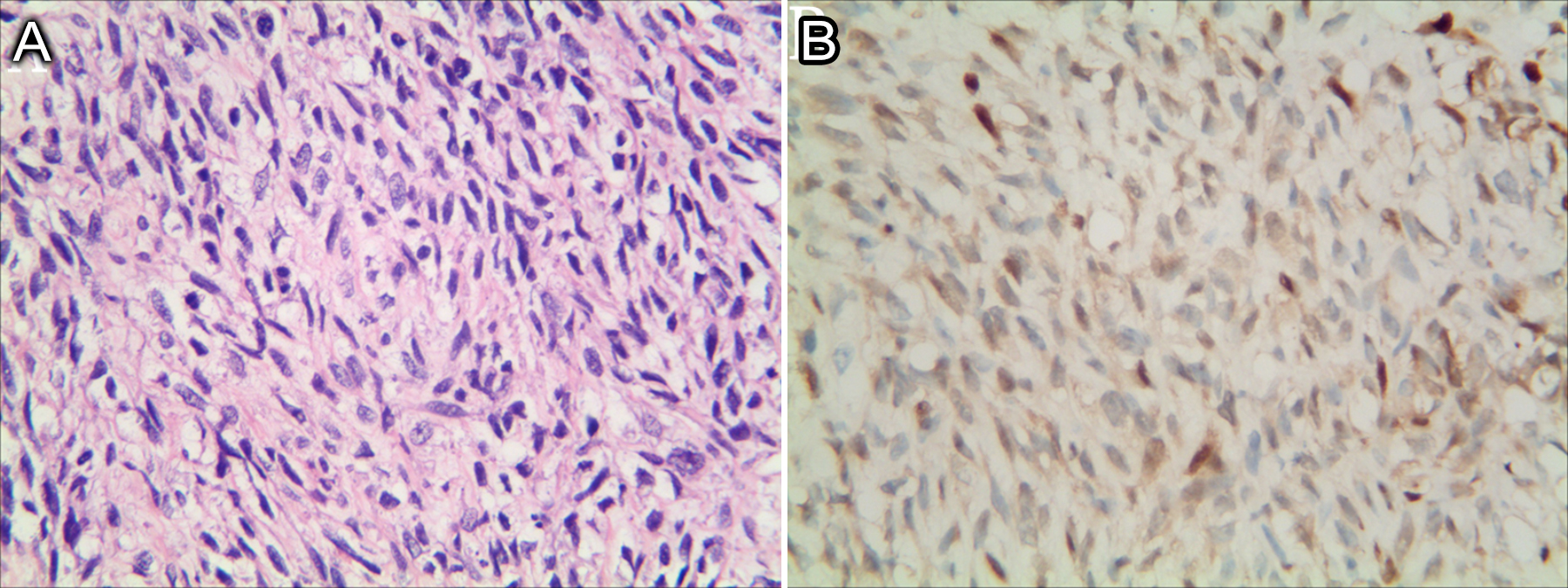 Figure 2: Histopathologic characteristics of the case. (A) Histology image showing a fascicular growth pattern composed of spindled cells (HE, ×200). (B) Immunohistochemistry showing positivity of tumor cells for S-100 (S-100, ×200).
Figure 2: Histopathologic characteristics of the case. (A) Histology image showing a fascicular growth pattern composed of spindled cells (HE, ×200). (B) Immunohistochemistry showing positivity of tumor cells for S-100 (S-100, ×200).
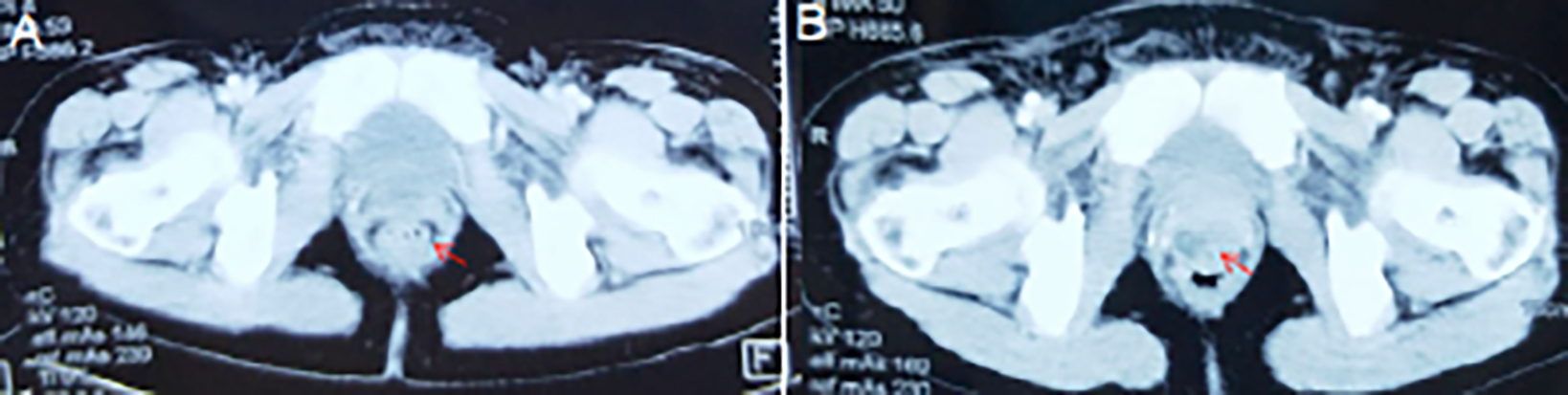 Figure 3: CT scan of the pelvis showing: (A) No recurrence of the tumor. (B) One year later, the patient was found to have a recto-vaginal fistula.
Figure 3: CT scan of the pelvis showing: (A) No recurrence of the tumor. (B) One year later, the patient was found to have a recto-vaginal fistula.
DISCUSSION
This case is reported because of its special location and treatment. This is a case of recurrent and metastatic cervical MPNST, in which, after recurrence and metastasis, epirubicin combined with ifosfamide chemotherapy has provided good results. Since the anthracycline drugs accumulated to the maximum dose, the patient was switched to dacarbazine to maintain chemotherapy. After the progression of the disease, the third and fourth line treatments were changed to etoposide combined with cisplatin, gemcitabine and docetaxel; however, the effect of chemotherapy was not satisfactory. The patient’s physical condition was poor. She could not tolerate continued chemotherapy, and therefore, she received targeted treatment with anlotinib till her death.
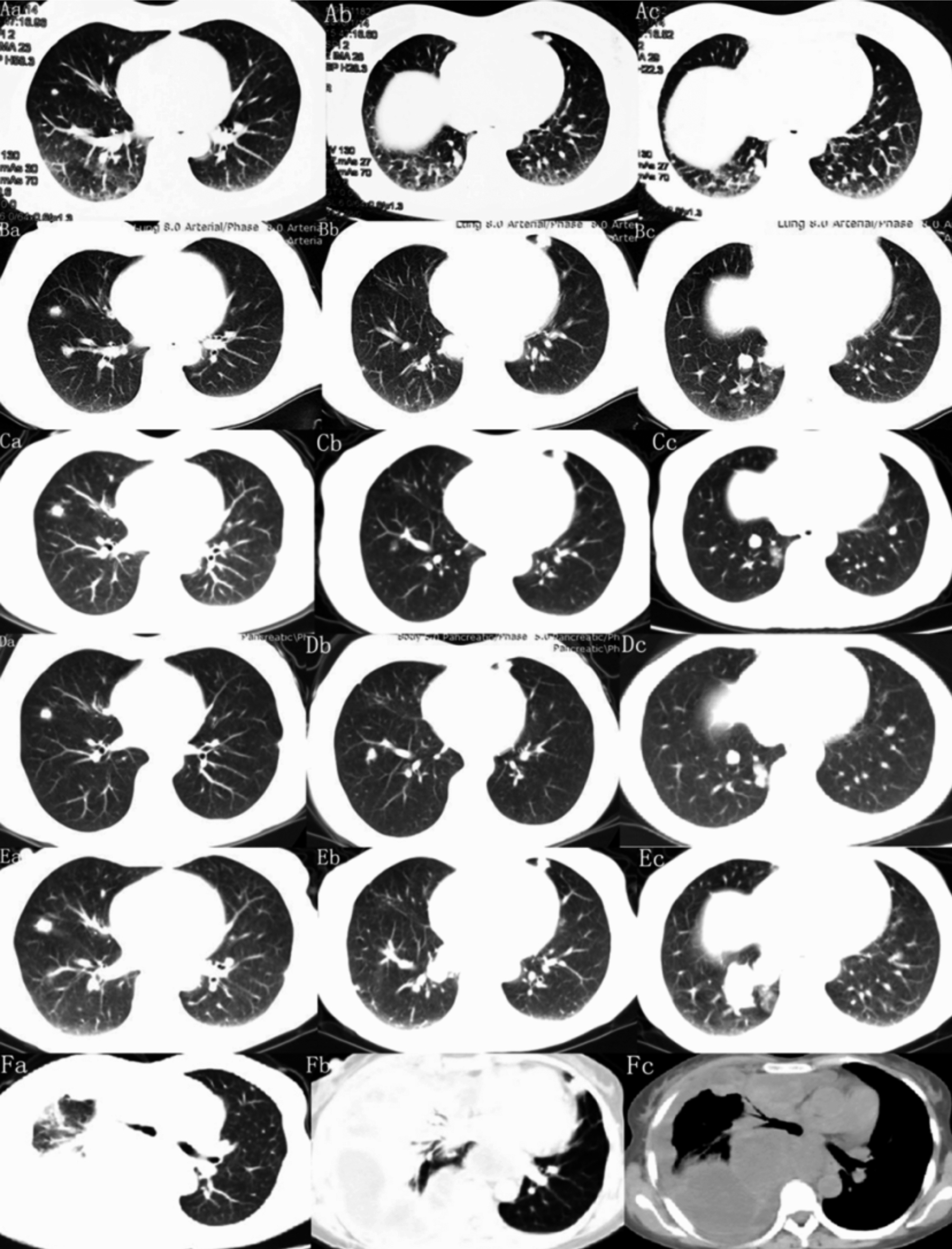 Figure 4: Changes of pulmonary metastases during chemotherapy. Axial CT images are shown corresponding to the treatment timeline. (A a,b,c) Baseline examination before first-line chemotherapy, and the pulmonary metastases are obvious. (B a,b,c) After 4 cycles of chemotherapy with docetaxel combined with gemcitabine. The lesions increased, and the disease progressed. (C a,b,c) After 8 cycles of AI chemotherapy, the metastatic lesions did not enlarge and no new metastases were found. (D a,b,c) After 2 cycles of Dacarbazine chemotherapy, the previous metastases did not increase, and no new metastases were found. (E a,b,c) After 3 cycles of dacarbazine chemotherapy, the marked lesions increased significantly and new metastatic foci appeared, and the disease progressed further. (F a,b,c) After one cycle of EP chemotherapy, right main bronchus obstruction, atelectasis, and pleural effusion were observed.
Figure 4: Changes of pulmonary metastases during chemotherapy. Axial CT images are shown corresponding to the treatment timeline. (A a,b,c) Baseline examination before first-line chemotherapy, and the pulmonary metastases are obvious. (B a,b,c) After 4 cycles of chemotherapy with docetaxel combined with gemcitabine. The lesions increased, and the disease progressed. (C a,b,c) After 8 cycles of AI chemotherapy, the metastatic lesions did not enlarge and no new metastases were found. (D a,b,c) After 2 cycles of Dacarbazine chemotherapy, the previous metastases did not increase, and no new metastases were found. (E a,b,c) After 3 cycles of dacarbazine chemotherapy, the marked lesions increased significantly and new metastatic foci appeared, and the disease progressed further. (F a,b,c) After one cycle of EP chemotherapy, right main bronchus obstruction, atelectasis, and pleural effusion were observed.
The diagnosis of MPNST depends on a combination of histology and immunohistochemistry. When a sarcoma meets one of the following criteria, it can be diagnosed as MPNST: (1) Origin from peripheral nerve; (2) malignant transformation of pre-existing benign neurofibroma; and (3) histology showing Schwann cell differentiation on immunohistochemistry or electron microscopy. The most common sites of MPNSTs are extremities (47%), trunk (35%), and head and neck (17%). This tumor is rarely found in the cervix.5 MPNSTs without NF-1 are very rare in the cervix. To date, only 10 cases have been reported in the literature. All of the 10 cases were without NF-1, and in none of them, metastasis or systemic chemotherapy was described. Lymphatic metastasis in MPNSTs is rare, and hematogenous spread is the most frequent route. The predominant location for metastases is the lung, accounting for 70% to 100% of all sites.6 Our patient developed recurrence and lung metastasis one year after the first radical resection, with a high degree of malignancy.
At present, the treatment of MPNSTs is similar to that of soft tissue sarcomas, and the prognosis mainly depends on the local tumor control. So far, the only effective treatment for MPNSTs is complete surgical resection. High doses of radiation have been recommended for tumors ≥5 cm and in the presence of microscopic positive margins.7 The use of adjuvant or neo-adjuvant chemotherapy in the treatment of soft tissue sarcomas, including MPNSTs, remains controversial. Studies have shown that chemotherapy is beneficial for survival and prevention of metastatic spread.6 Chemotherapy is part of comprehensive therapy; it can reduce the rate of distant metastases and appears to be more effective in controlling distant metastases. The combination of ifosfamide and doxorubicin is more effective than single-agent doxorubicin in the treatment of advanced or metastatic sarcoma, and is currently the preferred combination chemotherapy for soft tissue sarcomas.8 In retrospective analysis of EORTC trials, Kroep et al. found that doxorubicin-ifosfamide regimens are superior to other regimens for MPNSTs.9 In addition, Schuetze and Patel suggested that patients with large (>5 cm) and high-grade MPNSTs could be considered for chemotherapy, regardless of their location;10 the preferred chemotherapy regimen is a dose-intensive anthracycline and ifosfamide. Moretti et al. reported that the regimen of doxorubicin and ifosfamide, combined with surgery and radiation therapy, resulted in 57% disease-free survival and 80% overall survival (OS) at two years.6 The other phenomenon observed in the study by Moretti et al. was that no patient using doxorubicin and ifosfamide developed new metastatic disease throughout 25.4 months of the follow-up. The same phenomenon was observed in the study by Sordillo et al.,11 Some researchers have pointed out that chemotherapy can reduce the recurrence rate and distant metastasis and improve the quality of life, but does not prolong the OS of the patients. For the reported patient, the first choice after the diagnosis of cervical MPNST was a radical hysterectomy. After the operation, the patient was treated with epirubicin combined with isocyclophosphamide and cisplatin chemotherapy. One year later, local recurrence and lung metastasis occurred, and gemcitabine combined with docetaxel chemotherapy was administered. However, the disease was not well-controlled and continued to progress. Subsequently, eight cycles of AI chemotherapy were given, during which multiple imaging examinations were performed to evaluate the lung lesions. The results were consistent with the sensitivity of distant metastatic MPNSTs to the AI regimen. One of the dose-limiting toxicities of anthracyclines is cardiac toxicity. Since the accumulation of anthracyclines was maximal, in order to avoid cardiotoxicity, we considered dacarbazine as a second-line treatment. Dacarbazine was used to maintain chemotherapy for two cycles, but the disease was found to progress again.
In her case, the combination of surgery and multi-line chemotherapy allowed her to achieve the OS of 44 months. MPNSTs are not sensitive to chemoradiotherapy, but for advanced unresectable, metastatic, or recurrent tumors, comprehensive palliative care is recommended. Based on current relevant data, most researchers agree that chemotherapy plays an important role in patients with high-risk primary MPNSTs, who cannot undergo surgical resection, whose margins are positive, or who have metastases. However, this role still needs to be better defined through prospective, multi-institutional studies.
ETHICAL APPROVAL:
The report of this study was approved by the Ethics Committee of the Institution.
PATIENT’S CONSENT:
Written informed consent was obtained from the family member of the patient.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHOR’S CONTRIBUTION:
TZ: Performed the material analyses and wrote the manuscript.
ZW, RW: Helped perform the analysis with constructive discussions.
XW, MZ: Collected the clinical and imaging data of the patients.
FS: Selected topics and modified articles.
REFERENCES
- Mullins BT, Hackman T. Malignant peripheral nerve sheath tumors of the head and neck: A case series and literature review. Case Rep Otolaryngol 2014; 2014: 368920. doi: 10.1155/2014/368920.
- Evans DG, Baser ME, Mcgaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 2002; 39(5):311-4. doi: 10.1136/jmg.39.5.311.
- Guo A, Liu A, Wei L, Sonq X. Malignant peripheral nerve sheath tumors: differentiation patterns and immunohisto-chemical features: A mini-review and our new findings. J Cancer 2012; 3(1):303-9. doi: 10.7150/jca.4179.
- Stasik CJ, Tawfik O. Malignant peripheral nerve sheath tumor with rhabdomyosarcomatous differentiation (malig-nant triton tumor). Arch Pathol Lab Med 2006; 130(12): 1878-81. doi: 10.1043/1543-2165(2006)130[1878:MPNS TW]2.0.CO;2.
- Hwang IK, Hahn SM, Kim HS, Kim SK, Kim HS, Shin KH, et al. Outcomes of treatment for malignant peripheral nerve sheath tumors: Different clinical features associated with neurofibromatosis type 1. Cancer Res Treat 2016; 49(3): 717-26. doi: 10.4143/crt.2016.271.
- Moretti VM, Crawford EA, Staddon AP, Lackman RD, Oqilvie CM. Early outcomes for malignant peripheral nerve sheath tumor treated with chemotherapy. Am J Clin Oncol 2011; 34(4):417-21. doi: 10.1097/COC.0b013e3181e9c08a.
- Leachman BK, Galloway TJ. The role of radiation therapy in the management of sarcoma. Surg Clin North Am 2016; 96(5): 1127-39. doi: 10.1016/j.suc.2016.05.003.
- Edmonson JH, Ryan LM, Blum RH, Brooks JS, Shiraki M, Frytak S, et al. Randomised comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 1993; 11(7):1269-75. doi: 10.1200/ JCO.1993.11.7.1269.
- Kroep JR, Ouali M, Gelderblom H, Le Cesne A, Dekker TJ, Van Glabbeke M, et al. First-line chemotherapy for malignant peripheral nerve sheath tumor (MPNST) versus other histological soft tissue sarcoma subtypes and as a prognostic factor for MPNST: An EORTC soft tissue and bone sarcoma group study. Ann Oncol 2011; 22(1):207-14. doi: 10.1093/annonc/mdq338.
- Schuetze SM, Patel S. Should patients with high-risk soft tissue sarcoma receive adjuvant chemotherapy. Oncologist 2009; 14(10):1003-12. doi: 10.1634/theoncologist.2009- 0007.
- Sordillo PP, Helson L, Hajdu SI, Magill GB, Kosloff C, Golbey RB, et al. Malignant schwannoma-clinical characteristics, survival, and response to therapy. Cancer 1981; 47(10): 2503-9. doi:10.1002/1097-0142(19810515)47:10<2503: aid-cncr2820471033>3.0.co.