Liver Abscess in a Cyanotic Newborn
By Bengisu Guner Yilmaz1, Serdar Beken2, Eda Albayrak2, Metehan Ozen3, Ayse Korkmaz Toygar2Affiliations
doi: 10.29271/jcpsp.2022.Supp.S178ABSTRACT
Umbilical venous catheterisation is a common bedside procedure in the neonatal intensive care unit (NICU). Complications including thrombus formation, thromboembolism, vessel perforation, haemorrhage, and central line-associated bloodstream infection can be seen after the procedure. Pyogenic liver abscess is a rare but life-threatening complication of umbilical venous catheterisation. A male infant with cyanotic congenital heart disease was admitted to NICU. An umbilical venous catheter (UVC) was inserted. Abdominal X-ray showed an improperly positioned UVC, it was removed and replaced with a newer one. On the seventh day, the infant had abdominal distension and his clinical condition deteriorated. Abdominal ultrasonography (US) revealed a hepatic abscess. US-guided percutaneous abscess drainage was performed. Empiric antibiotic treatment was initiated and culture revealed Staphylococcus. The patient was followed by serial US and the lesion was completely resolved after three weeks. In conclusion, clinical worsening associated with gastrointestinal symptoms in a cyanotic infant with UVC should raise suspicion for liver abscess.
Key Words: Neonate, Hepatic abscess, Umbilical venous catheter, Cyanotic heart disease.
INTRODUCTION
Solitary pyogenic liver abscess in a neonate is a rare entity and has a high mortality rate.1 The risk factors are prematurity, low birth weight, total parenteral nutrition, intrauterine infections, previous antibiotic therapy, sepsis and interventions including umbilical vein catheterisation or surgery.2 Pathophysiology of hepatic abscess involves ascending infection via the umbilical and portal veins, hematogenous spread, spread by the biliary tree, and by direct contagious spread from neighbouring structures.3 Umbilical vein catheterisation is also a very important factor for central line-associated bloodstream infections (CLABSI), in terms of being the entry point of pathogens from the skin flora of the patient or hands of healthcare workers or contaminated fluids/drugs. Very few cases of liver abscess are reported in neonates with cyanotic congenital heart disease. Herein, we report a case of critical congenital heart disease (Taussig Bing anomaly and isthmus hypoplasia) and liver abscess in a neonate due to umbilical vein catheterisation.
CASE REPORT
A male neonate was born to a 31-year woman at 38 weeks of gestation by C-section as the first live-born child, weighing 3100 grams. The first and fifth minutes of Apgar scores were 7 and 8, respectively. There was no consanguinity between the parents. He was transferred to the neonatal intensive care unit (NICU) due to congenital heart disease diagnosed by fetal echocardiography at 24th weeks’ gestation.
On physical examination, the infant was cyanotic and oxygen saturation was 75-80% and there was a 2/6 systolic murmur. Other system examinations were normal. An echocardiographic study revealed Taussig Bing anomaly (Transposition of the great arteries, VSD-large subpulmonic) and transverse arcus-isthmus hypoplasia. The umbilical venous catheter (UVC) was immediately inserted and intravenous prostaglandin E2 treatment was initiated. To confirm the position of the UVC, a chest X-ray was performed and it was seen that UVC was improperly positioned to the liver (Figure 1). It was removed and replaced with the newer one in the correct position. Full enteral feeding was achieved on the third day of life and arterial switch surgery was planned for the following week. During this period, the infant’s oxygen saturation was between 75-85%.
On the seventh day of life, his clinical condition deteriorated and he developed abdominal distension and feeding intolerance. His laboratory tests were as follows: haemoglobin, 12.2 g/dl; haematocrit, 36.6%; leucocyte count, 18670/mm3; thrombocyte count, 344000/mm3; C-reactive protein (CRP), <0.05 mg/dl; procalcitonin, 13,3 ng/ml; Alanine aminotransferase (ALT), 120 IU/ml, and aspartate aminotransferase (AST), 184 IU/ml.
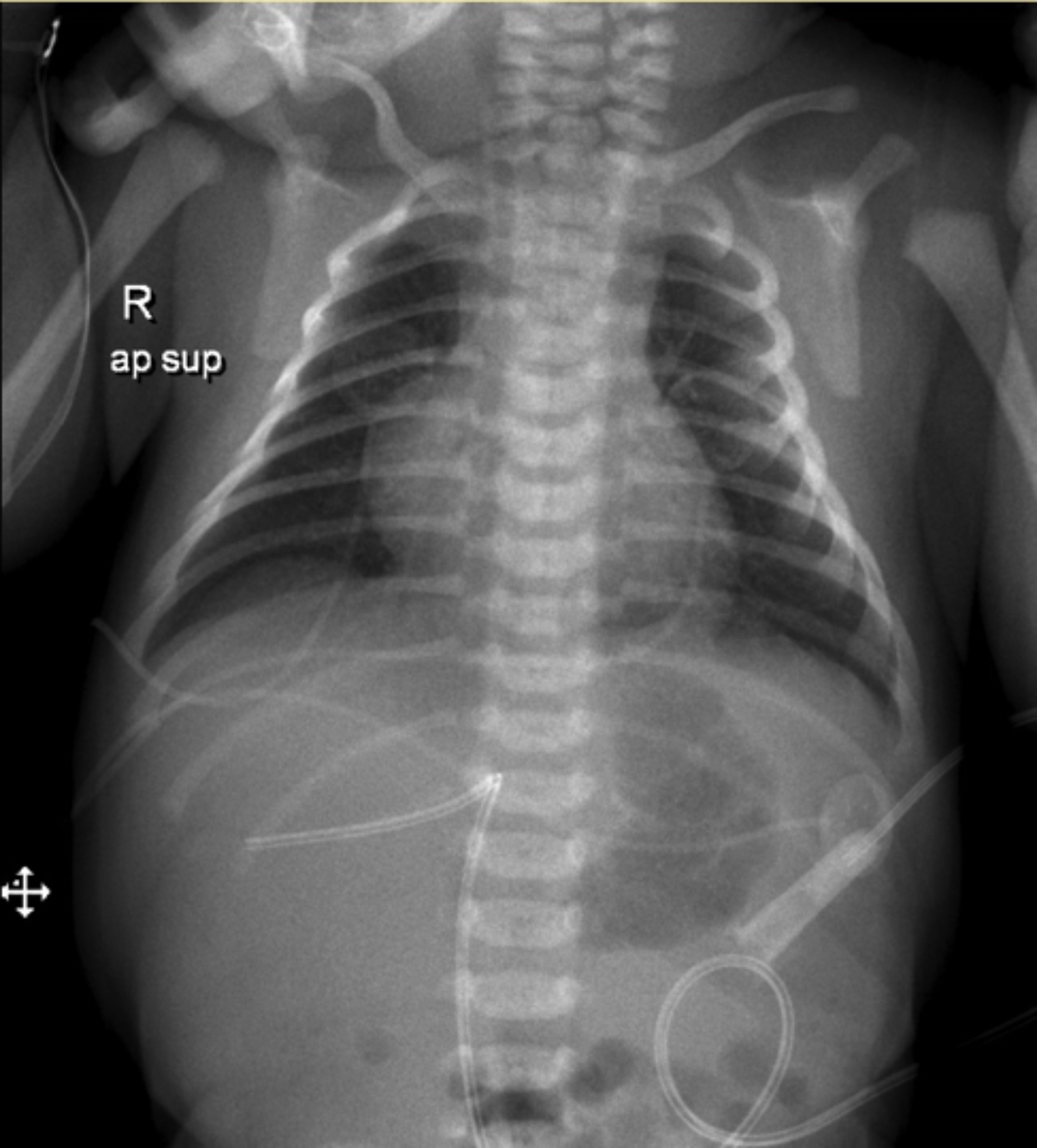 Figure 1: X-ray of the patient with improperly positioned umbilical vein catheter.
Figure 1: X-ray of the patient with improperly positioned umbilical vein catheter.
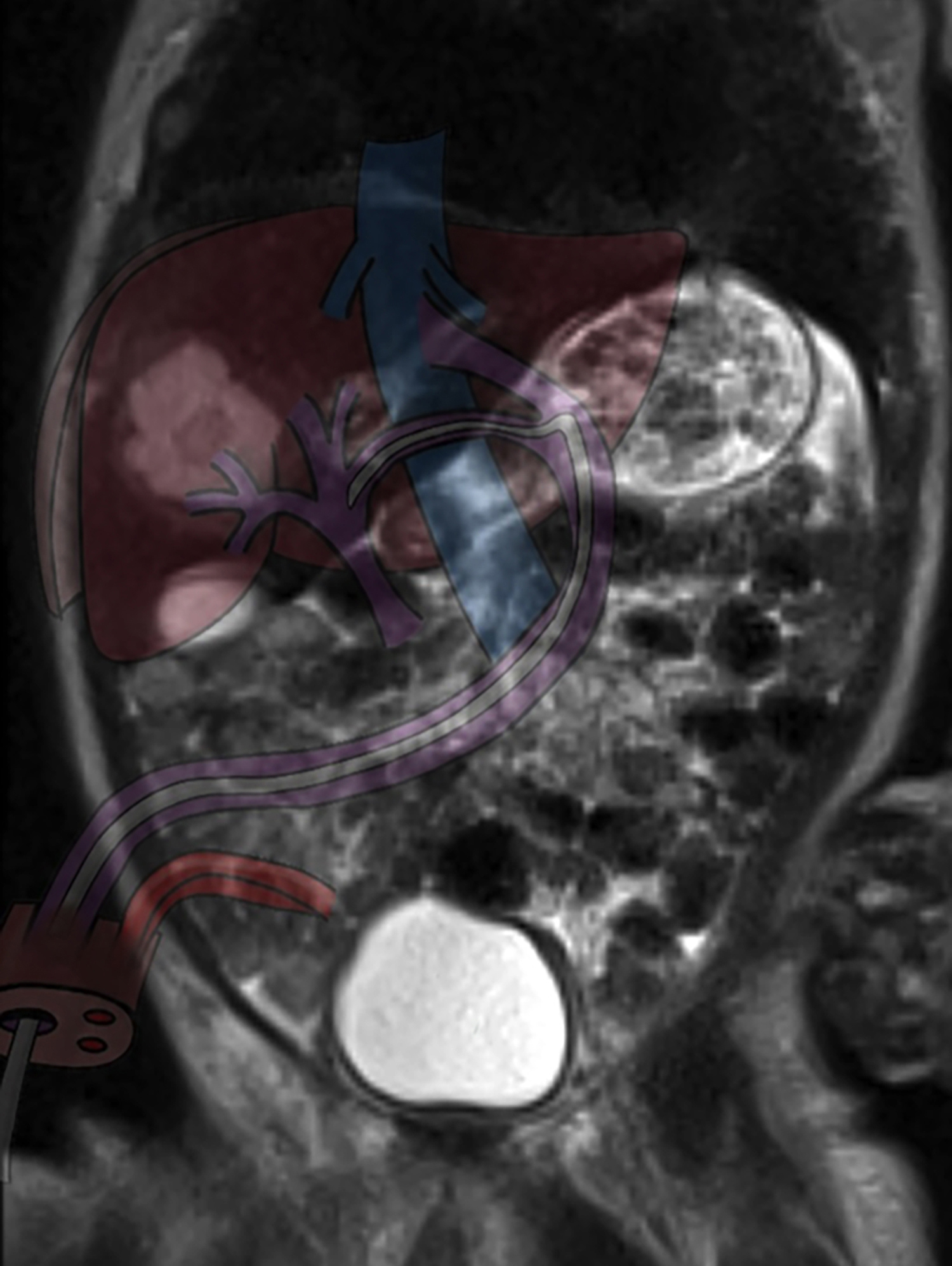 Figure 2: Illustration of malpositioned umbilical vein catheter on the coronal MRI section of the liver abscess.
Figure 2: Illustration of malpositioned umbilical vein catheter on the coronal MRI section of the liver abscess.
All cultures including central line blood culture were obtained. No growth was detected in the cultures. Because of abdominal distension, deranged liver function tests and procalcitonin, abdominal ultrasonography (US) was performed, which revealed a hypoechoic lesion with a lobulated contour in the anterior segment of the right lobe of the liver. Abdominal magnetic resonance imaging (MRI) was performed to rule out mass-occupying lesions. On MRI, 2.3×2.0 cm hyperintense, cystic lesion with peripheral contrast intake located medially to segment IV to V junction site, defined as abscess, was seen (Figure 2). US-guided percutaneous drainage was performed immediately and 3 ml purulent content was aspirated. Initially, suspecting early neonatal sepsis, empiric intravenous antibiotic (Vancomycin 40 mg/kg/day in 2 doses and Meropenem 20 mg/kg/day in 2 doses) therapy was initiated. In the microscopic examination of aspiration material, 8-10 leukocytes and gram-positive cocci were seen and culture revealed Staphylococcus aureus (MSSA), which was only resistant to penicillin. After the culture and antibiogram results, antibiotic treatment was switched to ampicillin-sulbactam, 100 mg/kg/day, in 4 doses and the treatment was completed in 3 weeks. Blood, cerebrospinal fluid (CSF) and urine cultures were negative. After drainage, the patient was followed by serial abdominal US and the lesion resolved gradually. Liver function tests and procalcitonin levels decreased. Neutrophil function tests, lymphocyte subsets, and immunoglobulin levels were all within normal ranges and possible major immune deficiency disorders were excluded.
He was successfully operated on postnatal 26th day and discharged on the 40th day of life. Control abdominal US was found to be normal prior to discharge and first-month follow-up.
DISCUSSION
The solitary pyogenic liver abscess has a non-specific clinical presentation and a high mortality rate in the neonatal period. Clinical signs and symptoms vary according to the accompanying clinical conditions, and are usually nonspecific and related to sepsis. The most common symptoms of hepatic abscess are fever, lethargy, vomiting, hepatomegaly, and abdominal distention.2,4 At least one positive haematological marker of sepsis (raised total white blood cell count, low platelet count or raised CRP/procalcitonin) can be the warning sign of infection, as in this case.
Mostly emphasised risk factors are umbilical vein catheterisation, previous antibiotic therapy, sepsis, necrotizing enterocolitis, surgery, prematurity, immune deficiency states, and parenteral nutrition1. Infants, especially low birth weight ones, are more susceptible to infections due to decreased adherence and chemotaxis of their neutrophils. In addition, high-risk infants exposed to invasive procedures such as umbilical catheterisation are vulnerable to CLABSIs, which is defined as an infection in a patient who had a central line insertion within 48 hours period before infection.5
UVC constitutes an entry point of pathogens from the skin flora of the patient or the hands of healthcare workers or contaminated fluids/drugs. In literature, incorrect placement of UVC and blood culture-proven neonatal sepsis are the most common predisposing factors for liver abscess.6 Umbilical vein catheterisation is one of the most commonly performed procedures in NICU for safe and reliable vascular access. After proper placement of UVC, intravenous fluids and medications can be administered safely. Malposition of UVC was reported to be nearly 50% at the first insertion attempt and correct position was reached only in 75% of the patients.7 On X-ray film, attention should be paid to the location of catheters. Neonatologists must be aware of malpositioned UVC, as the timely recognition and immediate management can improve the outcomes.
Another important factor, in this case, is that the infant had cyanotic congenital heart disease. It is well known that hypoxia affects the bacteria and the host interaction.8 Especially, in S. aureus infections, hypoxia induces abscess formation by blocking the access of host immune system via inhibiting the secretion of leucocidins and haemolysins to lyse neutrophils9. Grizelj et al., reported nine patients with severe liver injury due to UVC and of these seven were term-babies and one of them had hypoplastic left heart syndrome4. As in this case, not only low-positioned UVC, but also hypoxia seems to predispose the staphylococcal abscess formation. We suggest that cyanotic congenital heart disease is an additive risk factor for liver abscess development as in the present case.
Whenever possible, a liver abscess culture should be obtained for microbiologic diagnosis. Treatment may vary according to the size and number of abscesses, clinical status of the patient and causative agent. Small and multiple abscesses are managed conservatively using long-term antibiotics, whereas solitary types can be effectively managed by percutaneous aspiration or drainage.10 S. aureus, Streptococcus pyogenes, Escherichia coli, Klebsiella species (spp), Pseudomonas aeruginosa, Corynebacterium acnes, anaerobic microorganisms, and fungal pathogens like candida spp. have been reported in newborns with liver abscess and the most common causative agent is S. aureus.2 Regardless of the number or size of the lesions, empiric parenteral antibiotics should be initiated and an appropriate antibiotic regimen should be given for at least three weeks, depending on the patient’s clinical condition and the organism identified in the culture.
In conclusion, the hepatic abscess is a rare entity in the neonatal period but should be suspected in patients with critical congenital heart anomalies who show clinical deterioration and have undergone umbilical venous catheterisation previously.
PATIENT’S CONSENT:
The patient’s legal representatives gave written consent to publish this case report.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
BG, SB, EA, MO: Performed clinical follow-up of the patient.
BG, SB: Drafting of the manuscript.
AK, MO: Made the critical revision.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Semerci SY, Babayiğit A, Cebeci B, Büyükkale G, Çetinkaya M. Hepatic abscesses in preterm infants: Report of three cases and review of the literature. J Trop Pediatr 2016; 62(3):255-260. doi: 10.1093/tropej/fmv103.
- Rahman SU, Ghalib A, Abdulghani MH, Jan A. Liver abscess in a term baby: A case report and review of literature. Al Habib Medical J 2019; 1(1-2):27-9.
- Anand M, Sahi PK, Mantan M. Liver abscess in early infancy with no underlying risk factors: A case series. Trop Doct 2020; 51(2):49475520959937. doi: 10.1177/0049475 520959937.
- Grizelj R, Vukovic J, Bojanic K, Loncarevic D, Stern-Padovan R, Filipovic-Grcic B, et al. Severe liver injury while using umbilical venous catheter: Case series and literature review. Am J Perinatol 2014; 31(11):965-74. doi: 10.1055/ s-0034-1370346.
- Cho HJ, Cho H. Central line-associated bloodstream infections in neonates. Korean J Pediatrics 2019; 62(3): 79-84. doi: 10.3345/kjp.2018.07003.
- Singh A, Gupta A, Ansari M, Kumar S. Neonatal liver abscess with impending rupture presenting as abdominal wall swelling: A rare case. J Clinical Neonatology 2017; 6(4):270.
- Haase R, Hein M, Thale V, Vilser C, Merkel N. Umbilical venous catheters - analysis of malpositioning over a 10-year period. Z Geburtshilfe Neonatol 2011; 215(1):18-22. doi: 10.1055/s-0031-1271739.
- Schaffer K, Taylor CT. The impact of hypoxia on bacterial infection. FEBS J 2015; 282(12):2260-6. doi: 10.1111/febs. 13270.
- Hajdamowicz NH, Hull RC, Foster SJ, Condliffe AM. The impact of hypoxia on the host-pathogen interaction between neutrophils and staphylococcus aureus. Int J Mol Sci 2019; 20(22):5561. doi: 10.3390/ijms20225561.
- Lee SH, Tomlinson C, Temple M, Amaral J, Connolly BL. Imaging-guided percutaneous needle aspiration or catheter drainage of neonatal liver abscesses: 14-year experience. AJR Am J Roentgenol 2008; 190(3):616-22. doi: 10. 2214/AJR.07.2888.