Leukaemia Cutis
By Aamer Aleem, Farjah AlgahtaniAffiliations
doi: 10.29271/jcpsp.2023.05.592
Sir,
Leukaemia cutis (LC) is an uncommon manifestation of leukaemia in the skin. It is most frequently associated with acute myeloid leukaemia (AML).1 The frequency of LC may vary according to the type of leukaemia and is estimated to be between 3% to 5% of AML cases.2,3 LC is associated with a wide range of skin manifestations. The differential diagnosis of LC is broad and can include infectious, reactive, and neoplastic etiologies. A skin biopsy is needed in making a correct diagnosis. Systemic leukaemia usually presents concomitantly with LC, but cutaneous involvement may precede systemic leukaemia, making the diagnosis of LC more challenging.
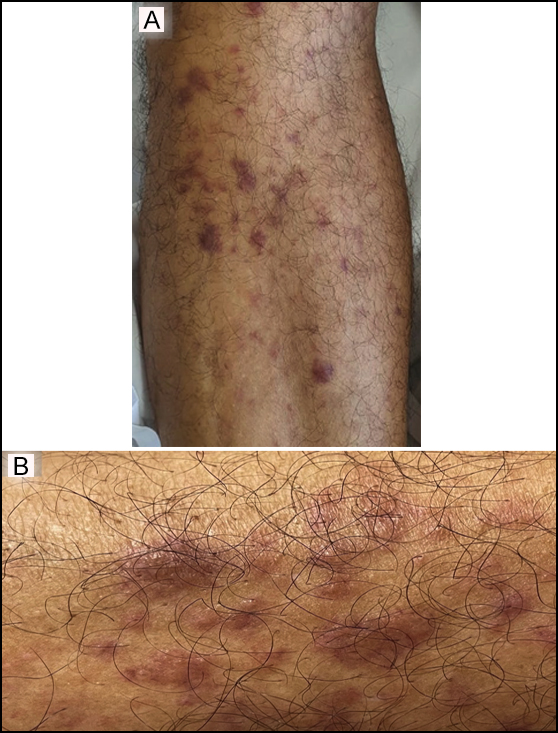 Figure 1: Leukaemia cutis lesions over the shin (A), and close up view (B).
Figure 1: Leukaemia cutis lesions over the shin (A), and close up view (B).
A 60-year previously healthy man presented to the emergency department with a 3-month history of fatigue, weight loss, and skin lesions over the body. On physical examination, he was noted to have pallor with violaceous papular/nodular, non-tender skin lesions of various sizes over the trunk, arms, and legs (Figures 1A, 1B). Largest lesion(s) measured 2 centimetres in the longest dimension. Laboratory studies revealed a white cell count of 8.6x109/L (reference range, 4.0 to 11.0 x109/L) with 4% blast cells on peripheral smear, low haemoglobin, and normal platelet count. Bone marrow (BM) findings were consistent with acute monoblastic leukaemia. The karyotype of the BM specimen revealed trisomy 8, and molecular analysis showed the presence of NPM1 and FLT3-ITD mutations. A skin biopsy confirmed infiltration of skin with leukaemia cells consistent with the diagnosis of leukaemia cutis (LC) (Figures 2A, 2B). Induction chemotherapy, along with sorafenib, an FLT3 inhibitor, was commenced. Skin lesions began to resolve after a few days and disappeared completely after 3 weeks. The patient achieved complete remission and underwent allogeneic-stem-cell-transplantation. He remains well 24 months post-transplant.
Pathogenesis of skin infiltration in LC remains unclear but as monocytic subtypes of AML are associated with extramedullary involvement, it may be a manifestation of the tendency to invade extramedullary tissues, including the skin. Adhesion molecules and chemokine receptors may have an important role in the skin homing of leukaemia cells.3 Some of the cytogenetic and molecular abnormalities found in patients with LC include translocation 8;21 [t(8;21)], trisomy 8, inversion 16 (inv 16), chromosome 11q23 rearrangements that involve the MLL gene, NPM1 mutation, and FLT3-ITD mutation.2,4
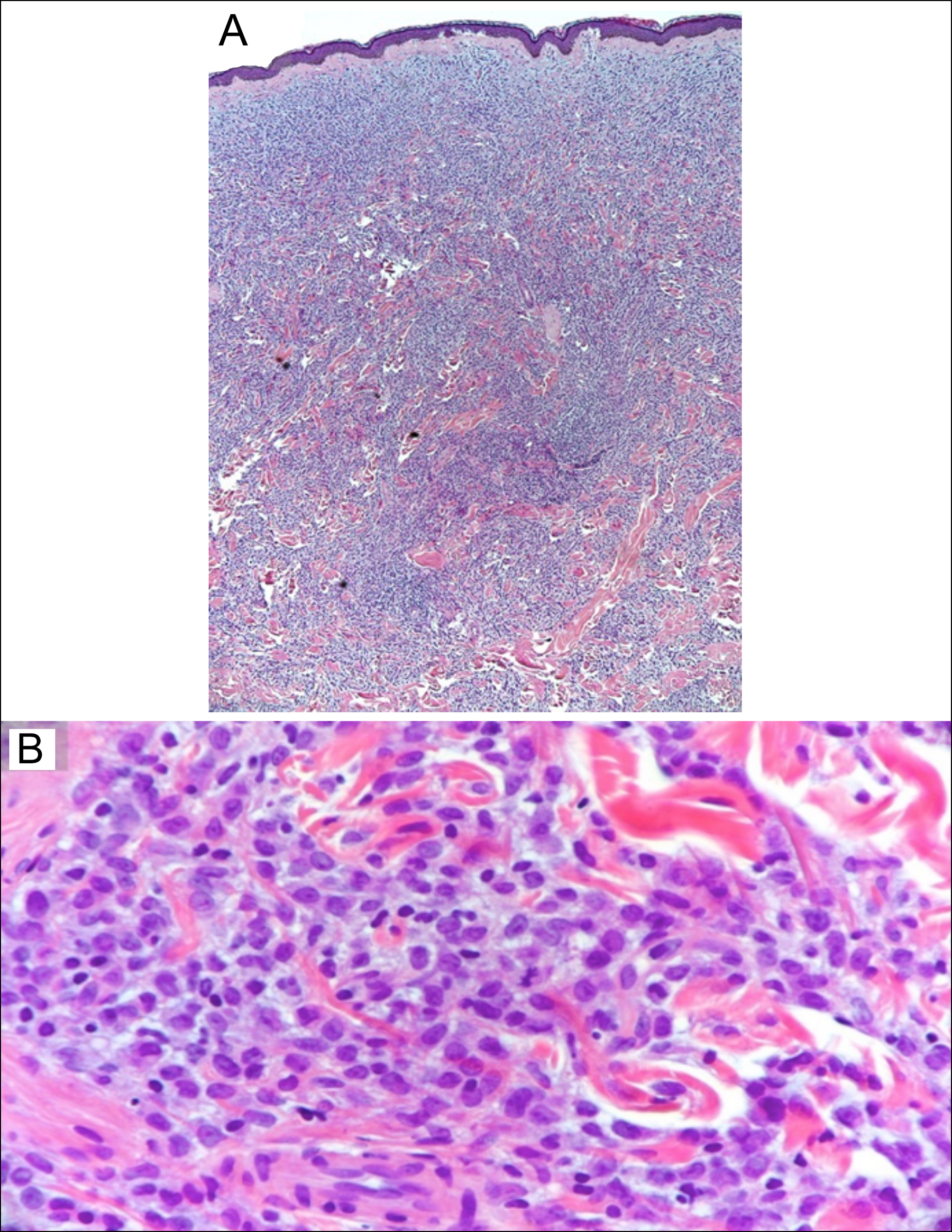 Figure 2: Photomicrograph of the skin biopsy specimen. (A) There is dense leukaemic cell infiltrate in the dermis with destruction of the collagen bundles on low-power view. (B) High-power view showing leukemic cell infiltration among the collagen bundles in the dermis.
Figure 2: Photomicrograph of the skin biopsy specimen. (A) There is dense leukaemic cell infiltrate in the dermis with destruction of the collagen bundles on low-power view. (B) High-power view showing leukemic cell infiltration among the collagen bundles in the dermis.
LC carries a poor prognosis as the extramedullary involvement in the skin and other areas may act as sanctuary sites for leukaemia cells and may not be cleared by chemotherapy.2,4 It is possible that the prognosis of patients with LC is determined by underlying cytogenetic and molecular abnormalities as some of these abnormalities like t (8;21) and NPM1 mutations, are associated with favourable prognosis, while trisomy 8 and FLT3-ITD are associated with poor prognosis.2,4 Intensive chemotherapy remains the standard treatment for fit patients with AML. Poor-risk patients are unlikely to be cured with chemotherapy alone, and if eligible, need to undergo allogeneic stem-cell transplant. With the availability of targeted agents like FLT3 inhibitors, the prognosis of these patients may improve.5
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
AA, FA: Clinical care of the patient, acquisition of the patient information and data, and manuscript writing.
Both the authors have approved the final version of the manuscript to be published.
REFERENCES
- Kaddu S, Zenahlik P, Beham-Schmid C, Kerl H, Cerroni L. Specific cutaneous infiltrates in patients with myelogenous leukemia: A clinicopathologic study of 26 patients with assessment of diagnostic criteria. J Am Acad Dermatol 1999; 40(6 Pt 1):966-78. doi: 10.1016/s0190-9622(99) 70086-1.
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol 2019; 155(7):826-32. doi: 10.1001/jamadermatol.2019. 0052.
- Agis H, Weltermann A, Fonatsch C, Haas O, Mitterbauer G, Mullauer L, et al. A comparative study on demographic, hematological, and cytogenetic findings and prognosis in acute myeloid leukemia with and without leukemia cutis. Ann Hematol 2002; 81(2):90-5. doi: 10.1007/s00277- 001-0412-9.
- Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol 2008; 129(1):130-42. doi: 10.1309/ WYACYWF6NGM3WBRT.
- Fiorentini A, Capelli D, Saraceni F, Menotti D, Poloni A, Olivieri A. The time has come for targeted therapies for AML: Lights and shadows. Oncol Ther 2020; 8(1):13–32. doi: 10.1007/s40487-019-00108-x.