Initial Experience in Ablation of Non-coronary Cusp PVCs by using High-density Mapping System (Rhythmia)
By Muhammad Asad, Qurban Hussain Khan, Azmat Hayat, Rehana KhadimAffiliations
doi: 10.29271/jcpsp.2022.04.6ABSTRACT
Idiopathic premature ventricular contractions (PVCs) most often occur under or above the semi-lunar valves from the right ventricular outflow tract and less often from the left ventricular outflow tract. Radio-frequency ablation is mostly used for patients who develop left ventricular dysfunction or intolerable symptoms. Since most of the patients are in the young age group and ablation has a high success rate with recurrence being relatively low, it can be offered to those symptomatic patients who want a definitive cure. The use of 3-dimensional (3D) electroanatomic mapping systems helps locate the ablation site. We report a case of a young man with PVCs coming from a rare site of non-coronary cusp, which was ablated successfully by using a high-density mapping system.
Key Words: Catheter ablation, Left ventricular outflow tract, Non-coronary aortic cusp, Premature ventricular contractions.
INTRODUCTION
Premature ventricular complexes (PVCs) are frequently found in the general population, commonly arising from the right ventricular outflow tract (RVOT) and left ventricular outflow tract (LVOT).1 Less common sites include the His-Purkinje system, fascicles, papillary muscle, pulmonary, and atrioventricular valves. Idiopathic PVCs can sometimes be seen in a repetitive monomorphic ventricular tachycardia (RMVT) pattern, characterised by frequent short runs of monomorphic, non-sustained ventricular tachycardias (VTs).2 LVOT PVCs are rarely present in the form of RMVT. Radiofrequency ablation (RFA) has been established as an effective treatment for outflow tract PVCs. Aortic sinus cusps (ASCs), anterior sites around mitral annulus (MA), and epicardium are the LVOT ventricular arrhythmias (VAs), identified in the literature.3 Rhythmia mapping system is a novel, rapid, high-density, non-fluoroscopic automatic mapping system which has been proved to be both safe and efficacious.4,5
This case is also one of such cases where LVOT PVC had left bundle branch block (LBBB) morphology and presented in RMVT fashion.
CASE REPORT
A 35-year man presented with complaints of palpitations and shortness of breath from 2007. Electrocardiograph (ECG) showed PVCs, for which he was prescribed β-blockers and calcium-channel blockers; but they still persisted. He presented to us with worsening of symptoms with ECG showing ventricular ectopics arising from LVOT based on LBBB morphology and V2 transition ratio > 0.6 (Figure 1).
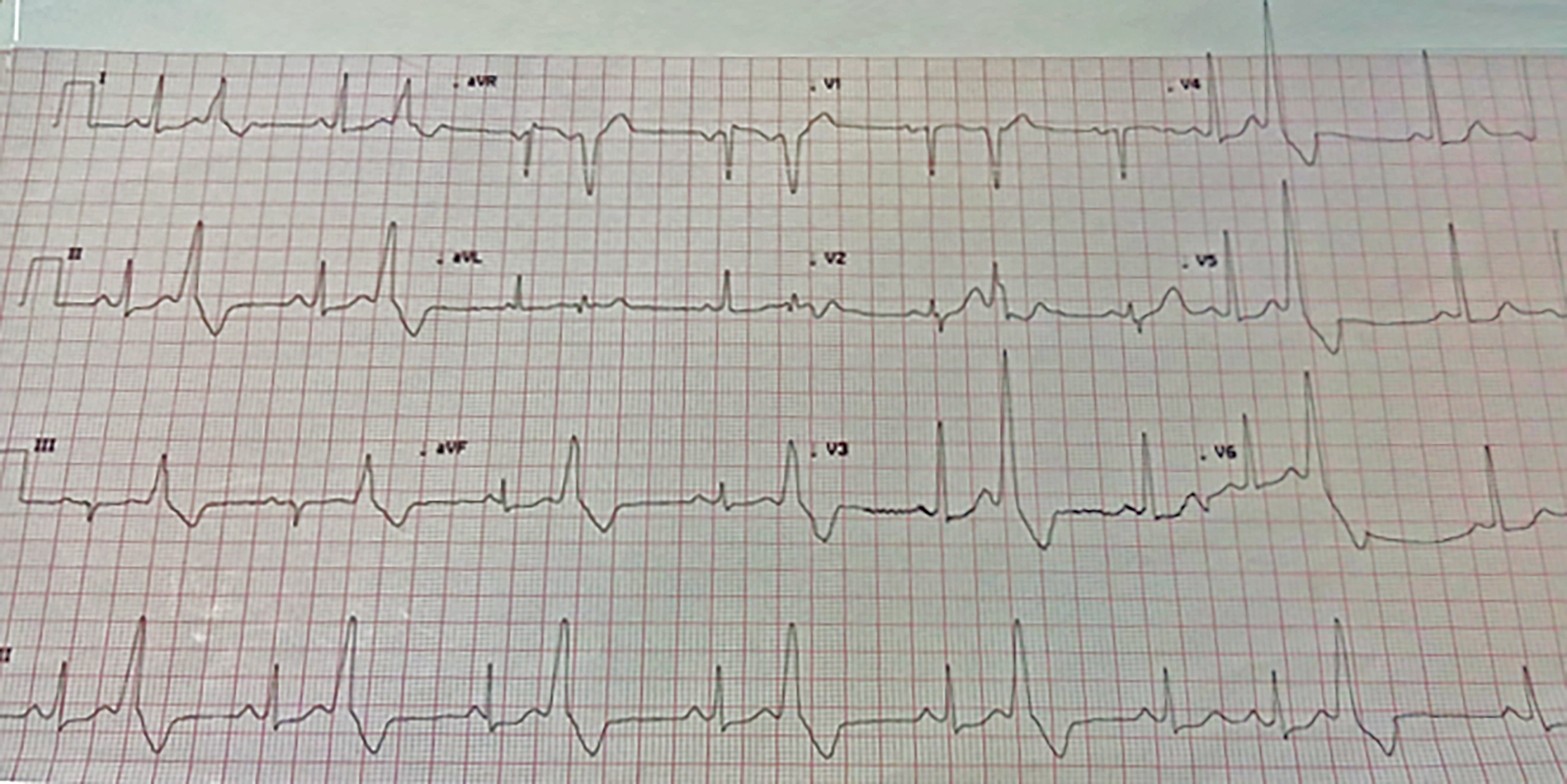 Figure 1: Premature ventricular complexes (PVCs) arising from region of left ventricular (LV) outflow tract (LVOT).
Figure 1: Premature ventricular complexes (PVCs) arising from region of left ventricular (LV) outflow tract (LVOT).
Holter showed a PVC burden of 33% and PVCs occasionally presented as RMVT form. The 2-D echocardiogram depicted ejection fraction (EF) of 55%, and no regional wall motion abnormality was present. His biochemical profile was normal, the ETT showed runs of non-sustained VTs, and coronary angiogram was normal. Despite using amiodarone and metoprolol for three months, PVCs did not settle, nor did they decrease in amount, so it was decided to do RFA of the patient.
The RFA was performed under sedation with local anesthesia. A 8.5F basket mapping catheter named Intellamap Orion, deployable basket electrode equipped with 64 electrodes, was introduced from the right femoral artery through a 9F vascular sheath via trans-aortic route. Rhythmia high-density mapping system was used.
Chamber geometry and electrograms (EGMs) were obtained by Orion, based on the beat acceptance criteria defined by the operator. The system incorporated unipolar (max negative dV / dt) and bipolar (max amplitude) EGMs for local activation annotation time. The mapping window was defined to concentrate on clinically relevant PVC components. The confidence mask method (points with bipolar EGM amplitude below the confidence mask in the immediate area have no color code and are shown in gray colour) was used to label an area as scar.
Activation mapping and pace mapping were used for PVC localisation. The goal was to have the local potential at least 20 milliseconds in front of the premature QRS wave and a unipolar signal with a QS wave. The earliest activation sites were then pace mapped in order to find the site of ideal match. A 12/12 match of the ECG pattern was finally seen at non-coronary aortic cusp anatomically, following which an open irrigated tip ablation catheter delivered RFA. This abolished PVCs but transiently, after which a new activation map was drawn as shown in Figure 2. A focus close to the same anatomical area of non-coronary cusp was found. This time, the target was a potential 33 msec ahead of the PVC on ablation catheter. Single ablation was done for two minutes, which abolished PVCs completely.
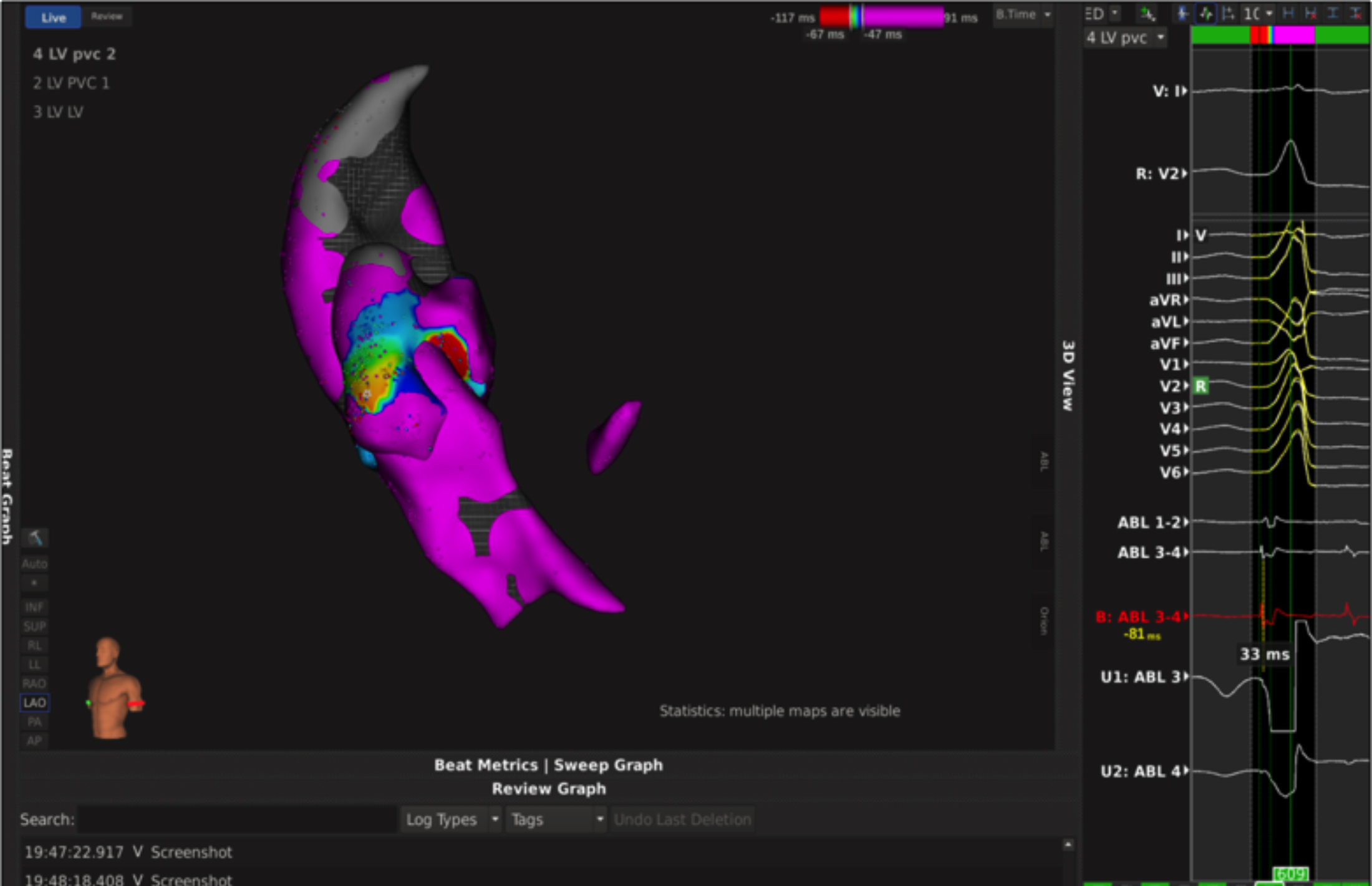 Figure 2: Left ventricular outflow tract (LVOT) activation map in the LAO projection. Red area in the representative EGM marking the site of earliest activation time (-33 msec) is shown.
Figure 2: Left ventricular outflow tract (LVOT) activation map in the LAO projection. Red area in the representative EGM marking the site of earliest activation time (-33 msec) is shown.
Burst pacing in left ventricle, both in the drug-free state and during continuous infusion of isoproterenol, failed to produce extra beats. The duration of the procedure was 2.5 hours. Fluoroscopy exposure and duration were 200 mGy and 10 minutes, respectively. The procedure and post-procedural in-hospital course were uneventful and the patient was discharged the next day. At follow-up at one month, he was asymptomatic and just 20 PVCs with different morphology from the previous one were recorded on 24 hours Holter.
DISCUSSION
PVCs are benign in structurally normal hearts, but the risk of cardiovascular failure, including stroke and mortality, is more than doubled in some studies.6,7 Idiopathic PVCs generally exit from RVOT and sometimes from the LVOT.1 The origin of LVOT VAs are known to exist in near anatomical vicinity.8 The RFA is considered a safe and effective treatment option for arrhythmias, but caution should be taken when attempting ablation as sensitive areas are present in this closely packed space.9 The non-coronary cusps’ (NCC) anatomical location is inferior, posterior and medial in comparison to other coronary cusps while the right coronary cusp (RCC) is placed anterior to NCC attached to interventricular septum, so the ECG and electrophysiological features of ventricular ectopics are similar to those of RCC VAs. In a study done by Yamada et al., it was observed that ectopics arising from NCC were rare and QRS morphology of this region has narrow QRS <150 ms along with an LBBB pattern and a smaller III / II ratio of 0.65. The present case corresponded to the NCCVA characteristics of the QRS morphologies previously reported.10
The anatomical relationship between outflow tracts and neighbouring cardiac structures is very important to understand. Since the PVCs can have different exit from the same anatomical area as was observed in our case, this phenomenon can be explained by understanding the aortic root's anatomy and electrophysiological properties of the aortic root. It is known from literature that ventricular tissue has crescents in the sinuses base, but in some populations, these extensions could expand beyond ventricle and arterial junction, and sometimes even to the region of semilunar valves. These sleeves can have a variable course, i.e., both oblique or longitudinally spread; they might be continuous or discontinuous and may exist outside the adventitia (epicardially). The arrhythmia focal origin may have different exit sites across the width of the semilunar valves due to the intriguing nature of such myocardial extensions.11
Electroanatomical system can be beneficial in such cases where one needs to be accurate in identifying the points of ablation, especially when there are important anatomical landmarks where ablation can be dangerous or even life-threatening.
PVCs arising from outflow tracts are seen commonly in the normal heart. The prognosis of such ectopics is considered favourable, but there is potential for PVC-related cardiomyopathy or even sudden cardiac arrest. For symptomatic patients, in which catheter ablation is indicated, the use of mapping systems help localise sites for successful ablation.
PATIENT’S CONSENT:
Informed consent was taken from the patient to publish this case.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
MA: Designed and authored the manuscript.
QHK: Literature review.
AH: Provided concept of the manuscript and proofread the final version before submission.
RK: Drafted the article, designed the work.
REFERENCES
- Noheria A, Deshmukh A, Asirvatham SJ. Ablating premature ventricular complexes: Justification, techniques and outcomes. Methodist Debakey Cardiovasc J 2015; 11(2): 109. doi: 10.14797/mdcj-11-2-109.
- Coggins DL, Lee RJ, Sweeney J, Chein WW, Hare GV, Epstein L, et al. Radiofrequency catheterablation as a cure for idiopathic tachycardia of both left andright ventricular origin. J Am Coll Cardiol 1994; 23(6):1333-41. doi: 10.1016/0735-1097(94)90375-1.
- Asirvatham SJ. Correlative anatomy for the invasive electrophysiologist: Outflow tract and supravalvar arrhythmia. J Cardiovasc Electrophysiol 2009; 20(8): 955-68. doi: 10.1111/j.1540-8167.2009.01472.
- Mantziari L, Butcher C, Kontogeorgis A, Panikker S, Roy K, Markides V, et al. Utility of a novel rapid high-resolution mapping system in the catheter ablation of arrhythmias: An initial human experience of mapping the atria and the left ventricle. JACC Clin Electrophysiol 2015; 1(5):411-20. doi: 10.1016/j.jacep.2015.06.002.
- Kennedy HL, Whitlock JA, Sprague MK, Kennedy LJ, Buckingham TA, Goldberg RJ. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Eng J Med 1985; 312(4):193-7. doi: 10.1056/NEJM198501243120401.
- Agarwal SK, Heiss G, Rautaharju PM, Shahar E, Massing MW, Simpson RJ. Premature ventricular complexes and the risk of incident stroke: The atherosclerosis risk in communities (ARIC) study. Stroke 2010; 41(4):588-93. doi.org/10.1161/STROKEAHA.109.567800.
- Ataklte F, Erqou S, Laukkanen J, Kaptoge S. Meta-analysis of ventricular premature complexes and their relation to cardiac mortality in general populations. Am J Cardiol 2013; 112(8):1263-70. doi: 10.1016/j.amjcard.2013.05.065.
- Callans DJ, Menz V, Schwartzman D, Gottlieb CD, Marchlinski FE. Repetitive monomorphic tachycardia from the left ventricular outflowtract: Electrocardiographic patterns consistent with a left ventricular siteof origin. J Am Coll Cardiol 1997; 29(5):1023-7. doi: 10.1016/s0735- 1097(97)00004-1.
- Zhu DW, Maloney JD, Simmons TW, Nitta J, Fitzgerald DM, Trohman RG, et al. Radiofrequency catheter ablationfor management of symptomatic ventricular ectopic activity. J Am Coll Cardiol 1995; 26(4):843. doi: 10.1016/0735-1097 (95)00287-7.
- Yamada T, Litovsky SH, Kay GN. The left ventricular ostium: An anatomicconcept relevant to idiopathic ventricular arrhythmias. Circ Arrhythmia Electrophysiol 2008; 1(5): 396-404. doi.org/10.1161/CIRCEP.108.795948.
- Hasdemir C, Aktas S, Govsa F, Aktas EO, Kocak A, Bozkaya YT, et al. Demonstration ofventricular myocardial extensions into the pulmonary artery and aortabeyond the ventriculo-arterial junction. Pacing Clin Electrophysiol 2007; 30(4):534-9. doi: 10.1111/j.1540-8159.2007.00704.x.