Inflammatory Pseudotumour of Temporal Bone in a Multiple-Comorbidity Patient
By Yao Wang, Chih-Hung Wang, Hsin-Chien ChenAffiliations
doi: 10.29271/jcpsp.2022.08.S168ABSTRACT
Inflammatory pseudotumour (IPT) of the temporal bone is relatively rare in the head and neck, but it is important for clinicians to be aware of this emerging entity. A 39-year man presented with a protruding reddish mass over the left external ear. High-resolution Computed Tomography and Magnetic Resonance Imaging of temporal bone showed soft tissue collection in the left external ear, middle ear, and mastoid cavity with bony erosion. The patient first received modified radical mastoidectomy. Several surgeries were required for recurrences with eventual intracranial invasion within 2 years. The pathology showed chronic inflammation without malignancy, autoimmune or infectious pathologies. Based on the clinical manifestations, IPT was diagnosed. Finally, radiation therapy (RT) with 30 Gy was given. There was no recurrence following the RT course. Early recognition of IPT presenting as a recurrent and locally aggressive inflammatory lesion in the temporal bone is necessary to achieve favorable outcomes.
Key Words: Inflammatory pseudotumour, Temporal bone, Radiation therapy.
INTRODUCTION
Inflammatory pseudotumour (IPT) is a benign, inflammatory lesion but may mimic malignant disease with aggressive and locally invasive behavior.1 The pathogenesis is still not well understood. It has been associated with previous inflammation, trauma, infection, surgery, and immune-mediated response.1,2 Although IPT occurs most commonly in the lung, it has also been detected in the liver, retroperitoneum, thorax, and abdominal viscera.3 IPT of the head and neck accounts for less than 5% of all extra-pulmonary cases with the orbit being the most common site.4 Temporal bone localization of IPT is rare; only less than 40 cases have been reported.1,4 Steroids and surgery are the most common treatment modalities, with occasional use of radiation or chemotherapy and immuno-modulation.1,4 We report a multiple-comorbidity patient complicated by a recurrent, aggressive, and locally invasive temporal bone inflammatory lesion with an ultimate favorable clinical outcome.
CASE REPORT
A 39-year man presented to our department with bloody otorrhea and hearing impairment of his left ear for several months. He had complicated medical histories of diffuse panbronchiolitis, chronic rhinosinusitis post-operation, type 2 diabetes mellitus (DM) with nephropathy, coronary artery disease post-stent placement, and dilated cardiomyopathy with congestive heart failure post-operation, and morbid obesity. Physical examination showed a protruding reddish mass occupying his left external ear canal (EEC) (Figure 1A). High-resolution Computed Tomography (HRCT) of temporal bone showed soft tissue collection in left EEC, middle ear cavity, and mastoid with bony erosion (Figure 1B). T2-weighted Magnetic Resonance Imaging (MRI) of the brain without contrast showed a large lesion extending from the mastoid to EEC with encasement of the dura (Figure 1C). At first, the patient received modified radical mastoidectomy and the pathology was diagnosed as pyogenic granuloma. Unfortunately, recurrence was noted three months later; modified radical mastoidectomy with mastoid obliteration was performed for second surgical management. One year later, the patient suffered from severe vertigo with vomiting. Repeat MRI showed recurrent lesion, which has expanded and invaded semicircular canals and otic capsule causing total hearing loss in the left ear. On surgical exploration, the lesion showed necrotizing material and granulation tissue occupying the left temporal bone region with severe erosion of posterior and superior semicircular canals, petrous apex, tegmen tympani and posterior cranial fossa. The lesion could not be completely removed. The patient underwent several debulking and debridement procedures for recurrences and intermittent subcutaneous abscesses of the skull within 2 years. During the course of follow-up, oral antibiotics and topical otic solutions were given for control. The histopathological examination showed acute and chronic inflammation without malignancy or infectious etiologies such as Tuberculosis (TB) and fungal infection (Figure 2). Autoimmune diseases such as Wegener’s granulomatosis and IgG4-related diseases were also excluded by laboratory examination and immunohistochemistry staining. The data showed negative antinuclear antibody titer and complement C3/C4 were within normal limits. Elevated erythrocyte sedimentation rate of 78 mm/hr (range reference: 0-15 mm/hr) and rheumatic factor of 57.5 IU/ml (range reference: < 20 IU/ml) were shown. The titer of cytoplasmic-antineutrophil cytoplasmic antibody (ANCA) and perinuclear-ANCA were less than 0.2 IU/ml (range reference: <2 IU/ml). IHC staining revealed less than 1% infiltration of IgG4-positive plasma cells in the lesion with mild elevated plasma IgG4 levels of 100.4 mg/dl (reference range: 3.9-86.4 mg/dl). However, a recent MRI showed intracranial invasion over the cerebellum and dura infiltration (Figure 3). Based on previous surgical results, the lesion was not easily accessible and could not be completely removed. In view of the clinical manifestations and histopathology result, the diagnosis of IPT of the temporal bone was rendered in this patient. We did not give steroids to the patient due to poor control of DM. Alternatively, radiation therapy (RT) with total 30 Gy (200 cGy/fraction) was given. Dramatically, the lesion in the mastoid cavity subsided and the cavity showed gradual epithelialisation after RT (Figure 4). Three months after RT, MRI scans showed the disappearance of the intracranial lesions. There was no recurrence during one-year follow-up post-RT.
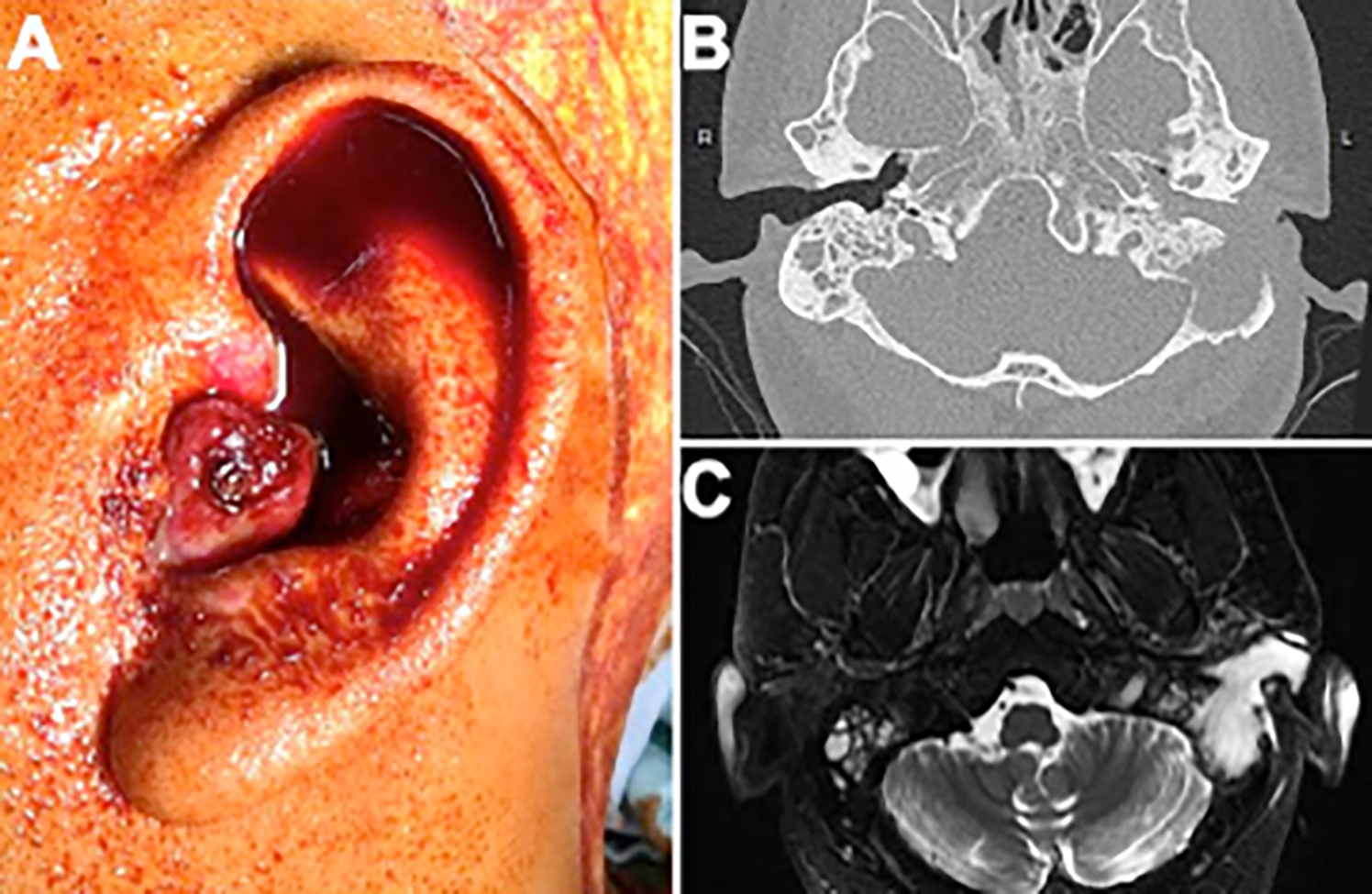 Figure 1 (A): A view of the tumor extending to the external ear as a reddish polypoid lesion. (B) Initial imaging by High-resolution Computed Tomography (HRCT) of temporal bone showed soft tissue lesion in left EEC, middle ear cavity and mastoid with bony erosion. (C) Magnetic Resonance Imaging (MRI) of head and neck showed a large lesion extending from mastoid to EEC with encasement of the dura.
Figure 1 (A): A view of the tumor extending to the external ear as a reddish polypoid lesion. (B) Initial imaging by High-resolution Computed Tomography (HRCT) of temporal bone showed soft tissue lesion in left EEC, middle ear cavity and mastoid with bony erosion. (C) Magnetic Resonance Imaging (MRI) of head and neck showed a large lesion extending from mastoid to EEC with encasement of the dura.
DISCUSSION
We presented a multiple-comorbidity patient complicated by a recurrent, inflammatory, aggressive, and locally invasive temporal bone lesion. The clinical manifestations and histopathology were suggestive of IPT, and the lesion was finally eradicated using RT.
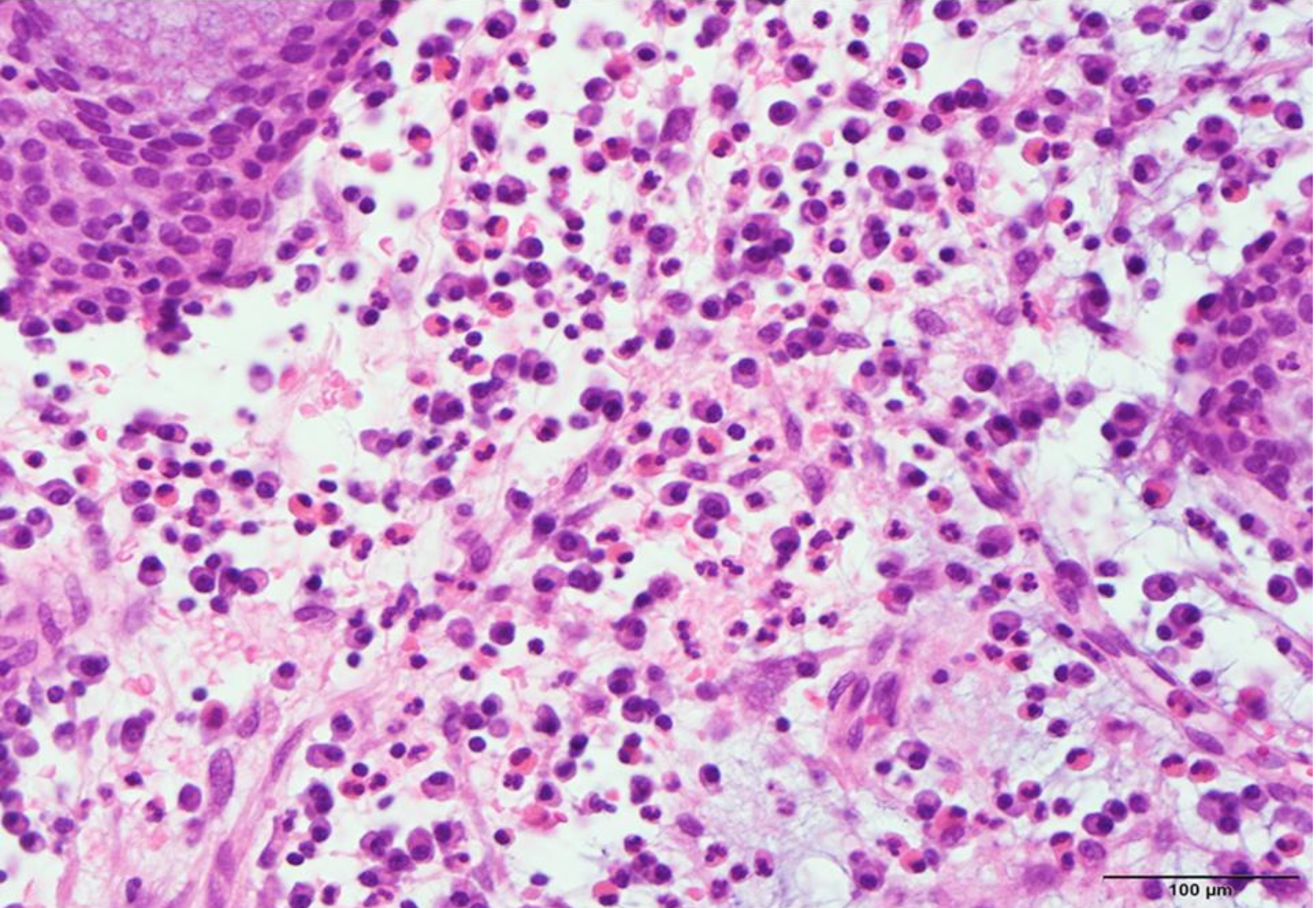 Figure 2: The histopathology showed prominent eosinophils and plasma cells infiltration in an edematous and fibrous background. (HE, ×400).
Figure 2: The histopathology showed prominent eosinophils and plasma cells infiltration in an edematous and fibrous background. (HE, ×400).
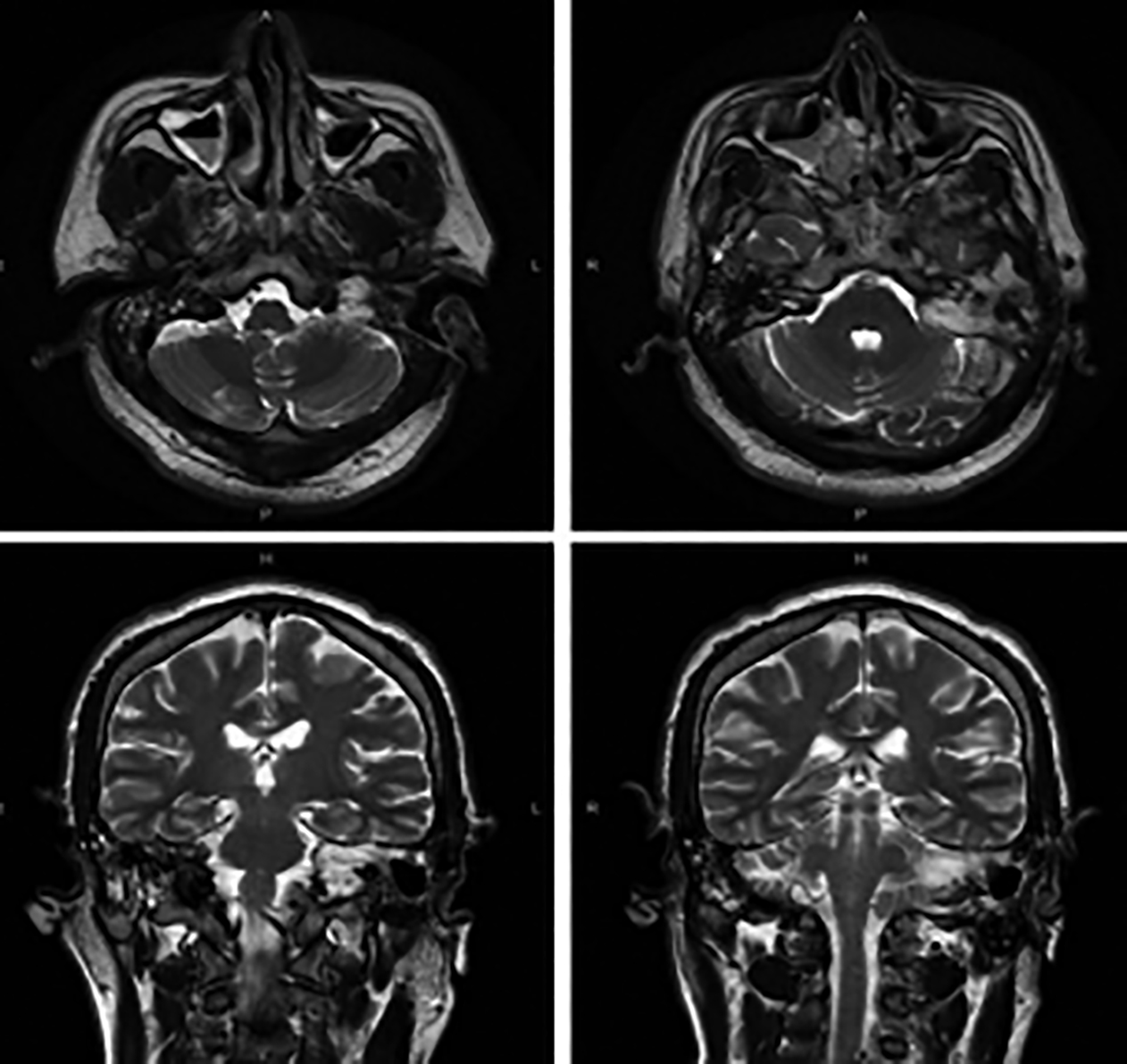 Figure 3: Postoperative axial and coronal T2-weighted Magnetic Resonance Imaging (MRI) without contrast revealing marked involvement of petrous apex and cerebellum.
Figure 3: Postoperative axial and coronal T2-weighted Magnetic Resonance Imaging (MRI) without contrast revealing marked involvement of petrous apex and cerebellum.
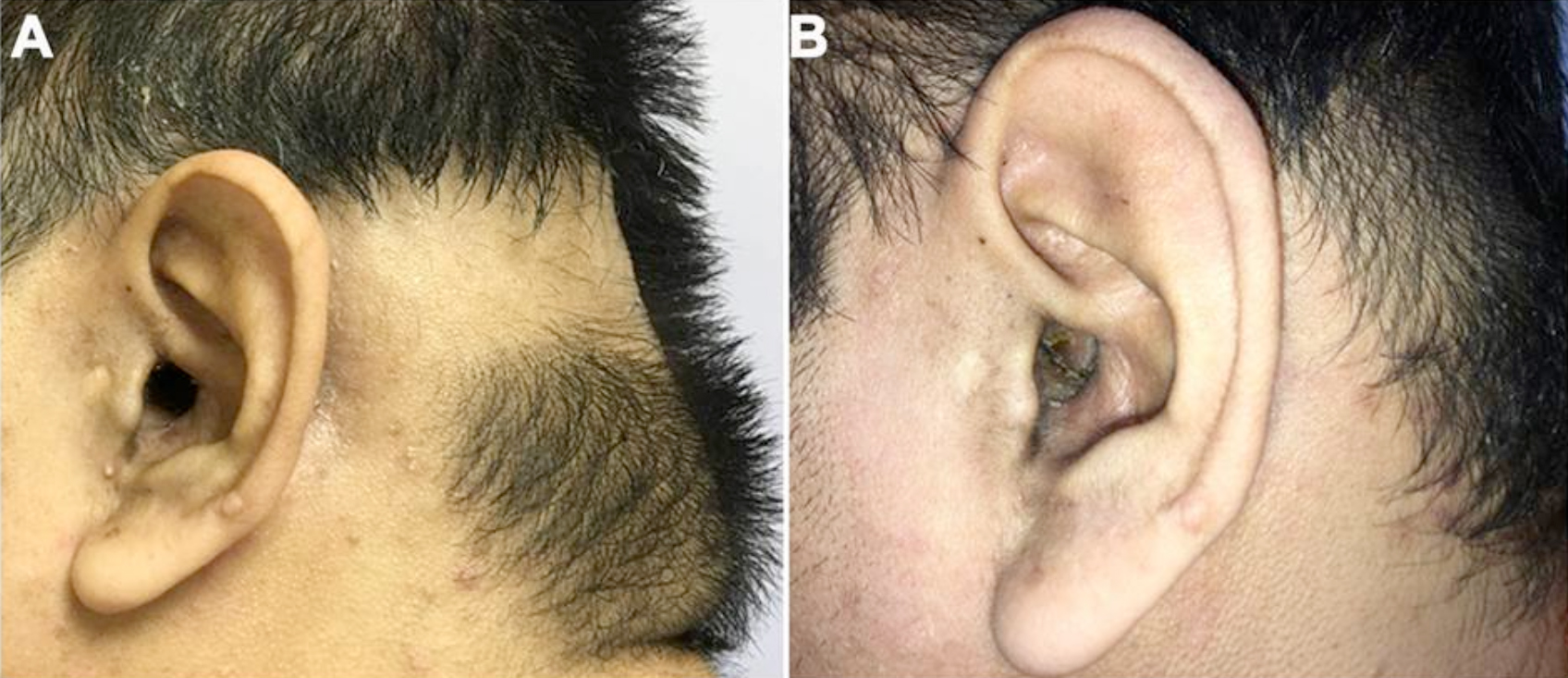 Figure 4: (A) After radiation therapy, alopecia was noted and the lesion had shrunken in size. (B) Three months later, the cavity of mastoid became well-epithelialised without the lesion and the hair had regrown.
Figure 4: (A) After radiation therapy, alopecia was noted and the lesion had shrunken in size. (B) Three months later, the cavity of mastoid became well-epithelialised without the lesion and the hair had regrown.
IPTs account for approximately 5% of the cases in the head and neck; however, it is important for clinicians to be aware of this disease process because the entity has been increasingly recognised during recent past.2-4 Temporal bone IPT is benign and relatively rare amongst the head and neck lesions presenting as isolated or multiple lesions, but possesses an aggressive and locally invasive behavior.1,2,5
An early diagnosis and management are important to avoid significant morbidity. Biopsy is the gold standard for the diagnosis of IPT as malignancy must be excluded for all suspicious lesions.6 It is histologically composed of myofibroblastic spindle cells with lymphocytes and plasma cells infiltration with fibrosis, necrosis, and granulomatous reaction. The etiology of IPT remains unclear but immunological and infectious causes have been postulated.2 While MRI often demonstrates iso- or hypo-intense soft tissue lesions on T1-weighted images, and marked hypo-intensity on T2-weighted images, the radiologic features of IPT are variable and nonspecific.7,8 Our case showed hyper-intense lesions on T2-weighted MRI images.
Treatments include multiple therapeutic modalities based on the patients’ symptoms, physical condition and neurologic disability.2 Surgery is the mainstay of treatment in a majority of cases. Patients who recur or are unable to tolerate steroids or inaccessible diseases or poor surgical candidates may require additional therapies. Approximately 20% of approximately 40 reported cases with temporal bone IPTs were treated with radiation as an adjunctive therapy.1 Our case also showed complete remission after RT. Immuno-modulation is currently being favored with Rituximab for intracranial extension and multifocal IPTs over radiation.1,4
Our case had multiple comorbidities such as heart failure and severe respiratory compromise which lead to some hesitation and delay of treatment for temporal bone IPT due to surgical and anesthetic safety considerations. Early recognition of IPT presenting as a recurrent and locally aggressive inflammatory lesion in the temporal bone may result in favorable outcomes, but long-term follow-up is necessary.
PATIENT'S CONSENT:
The informed consent was obtained from the patient for publication.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
YW: Collected data and drafted the manuscript.
CHW: Critically review the manuscript.
HCC: Edited the manuscript and gave final approval to the collected data.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Sakano H, Shih CP, Jafari A, DeConde A, Harris JP. Multifocal inflammatory pseudotumor of the temporal bone, maxillary sinus, and orbit. Otol Neurotol 2018; 39:e1125-e8.
- Tian H, Liu T, Wang C, Tang L, Chen Z, Xing G. Inflammatory pseudotumor of the temporal bone: Three cases and a review of the literature. Case Rep Med 2013; 2013:480476. doi: 10.1155/2013/480476.
- Strasnick B, Vaughan A. Inflammatory pseudotumor of the temporal bone: A case series. Skull Base 2008; 18(1):49-52. doi: 10.1055/s-2007-993047.
- Ortlip TE, Drake VE, Raghavan P, Papadimitriou JC, Porter NC, Eisenman DJ, et al. Inflammatory pseudotumor of the temporal bone: A case series. Otol Neurotol 2017; 38:1024-31. doi: 10.1097/MAO.0000000000001465.
- Lee JH, Jung MK, Song CE, Yeo SW, Lee HK, Yang PS, et al. Concomitant inflammatory pseudotumor of the temporal bone and lung: A case report. Ear Nose Throat J 2007; 86(10):614-16.
- Williamson RA, Paueksakon P, Coker NJ. Inflammatory pseudotumor of the temporal bone. Otol Neurotol 2003; 24(5):818-22. doi: 10.1097/00129492-200309000-00021.
- Han MH, Chi JG, Kim MS, Chang KH, Kim KH, Yeon KM, et al. Fibrosing inflammatory pseudotumors involving the skull base: MR and CT manifestations with histopathologic comparison. AJNR Am J Neuroradiol 1996; 17(3):515-21.
- Park SB, Lee JH, Weon YC. Imaging findings of head and neck inflammatory pseudotumor. AJNR Am J Neuroradiol 2009; 193(4):1180-6. doi: 10.2214/AJR.09.2398.