Histiocytic Sarcoma with Invasive Urothelial Carcinoma of Urinary Bladder
By Aysegul Isal Arslan1, Sevil Karabag2, Murat Akgul2Affiliations
doi: 10.29271/jcpsp.2022.04.79ABSTRACT
We report a rare case of histiocytic sarcoma in association with invasive urothelial carcinoma involving the urinary bladder. A 67-year male patient, who presented with a complaint of macroscopic hematuria, was found to have a mass on urinary system ultrasonography. Abdominal magnetic resonance imaging was performed to evaluate the mass, which showed a 52×24 mm mass on the posterior wall of the bladder. The cystoscopic examination revealed two suspicious areas in close proximity to one another with solid-papillary character. The tissue samples were collected by means of transurethral resection. The evaluation of these samples revealed two distinct neoplastic patterns. The areas of invasive urothelial carcinoma infiltrating lamina propria were noted on the surface in addition to diffuse sheets of large cells with hyperchromatic nuclei and ample clear cytoplasm, with a patternless pattern among small lymphocytes in myxoid background in the lamina propria. The positive reactions were observed in these areas with CD45, fascin, and CD68, a histiocytic marker. The histopathological diagnosis was histiocytic sarcoma in combination with invasive urothelial carcinoma. Histiocytic sarcoma may mimic several other malignant lesions, and only immunohistochemistry can identify this tumour to allow correct treatment. We present this rare case to emphasise that this phenomenon should be considered in unusual tumors and sites.
Key Words: Urinary bladder, Histiocytic sarcoma, Invasive urothelial carcinoma.
INTRODUCTION
Histiocytic neoplasms are rare tumours responsible for <1% of lymph node tumours originating from mononuclear phagocytes (macrophages and dendritic cells) or histiocytes. Since many of these tumour types have not been recognised until recently, their true incidence currently remains unknown. Although these tumours may be seen in any age group, but most of the cases have been observed in adults, with a mean age of 52 years.1
Non-urothelial neoplasms of the urinary bladder account for less than 5% of all bladder tumours in North America.2 The literature shows that approximately half of the bladder sarcomas are leiomyosarcomas and the rest consist of rhabdomyosarcomas, angiosarcomas, osteosarcomas and carcinosarcomas.3,4 Histiocytic sarcoma is a fairly rare malignant neoplasm with only a limited number of extranodal cases reported to date.
When the disease occurs in an extranodal region, the most commonly involved sites appear to be the intestinal system, skin and soft tissue. Certain cases may manifest as low-grade cancers in a synchronous or metachronous pattern, particularly, with follicular lymphoma and chronic lymphocytic leukaemia/small lymphocytic lymphoma. There is only a limited number of case reports in the literature showing the presence of histiocytic sarcoma in the urinary system.5 Interestingly, we have identified several veterinary cases in the literature. This tumour appears to occur more frequently in lymph nodes of animals, especially in cats and dogs.6,7
In this report, we present a case of extranodal histiocytic sarcoma in the urinary bladder with a highly rare urological localisation accompanied by invasive urothelial carcinoma in the light of relevant literature.
CASE REPORT
A 67-year male patient presented to the urology clinic with a complaint of macroscopic haematuria. His urinary system ultrasonography revealed a mass measuring 44×19 mm localised on the posterior wall of the bladder. The magnetic resonance imaging of the lower abdomen showed a 52×24 mm mass on the posterior wall of the bladder (Figure 1).
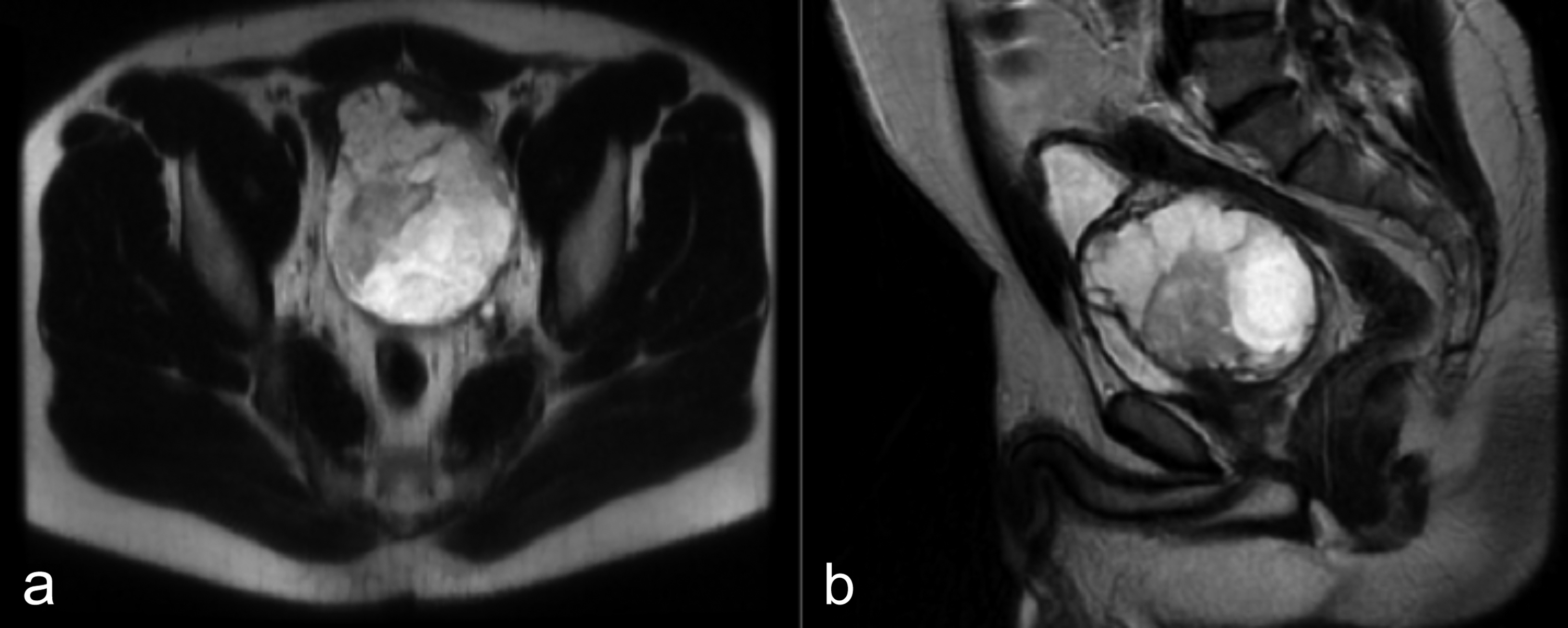 Figure 1: Preoperative magnetic resonance imaging of the tumor; (a) Transverse section; (b) Sagittal section.
Figure 1: Preoperative magnetic resonance imaging of the tumor; (a) Transverse section; (b) Sagittal section.
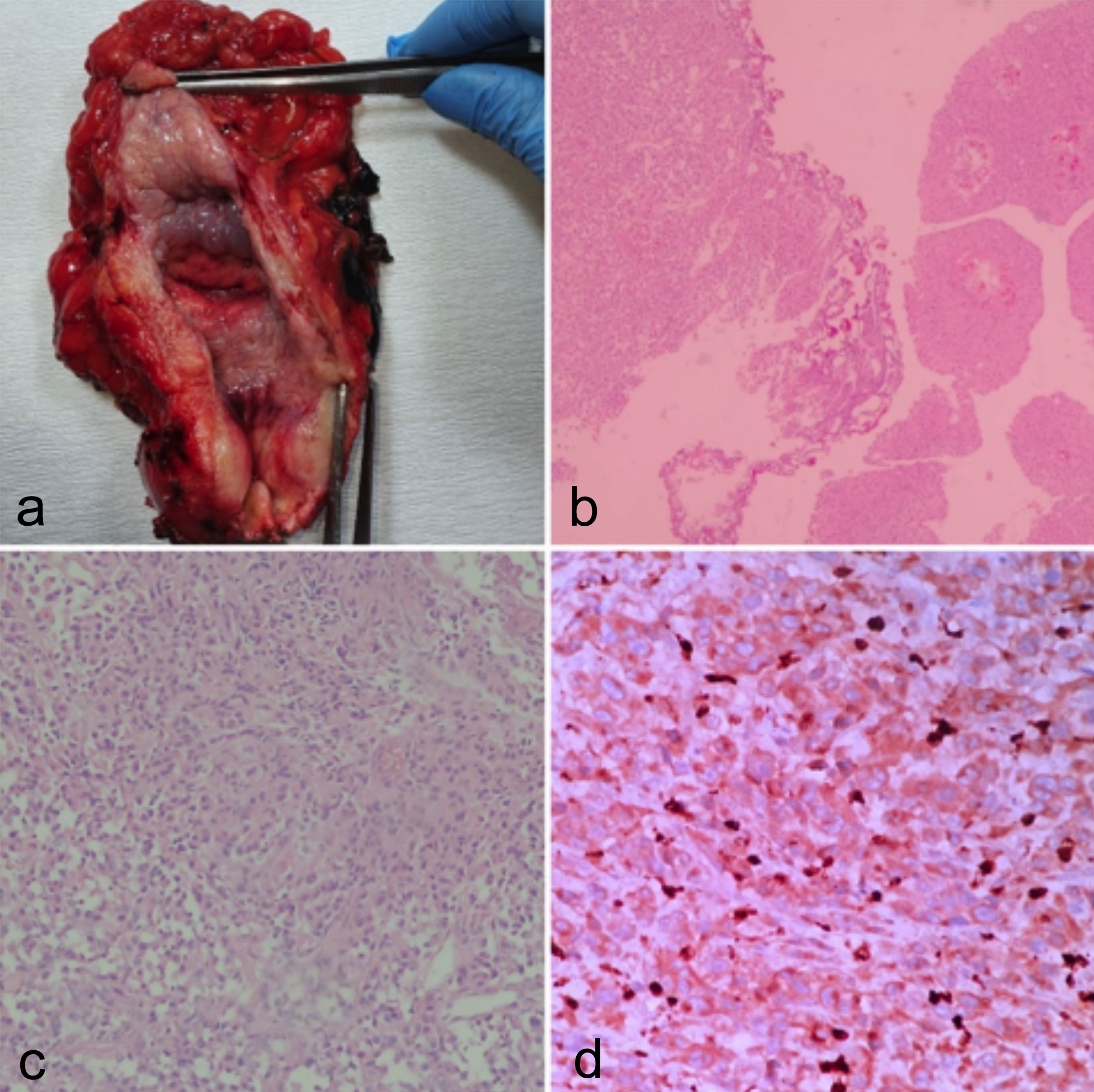 Figure 2: (a) Gross appearance of cystectomy specimen; (b) Low-power view showing papillary urothelial neoplasm and invasive nests of tumor in lamina propria (H&E, ×200); (c) High-power view showing the focus of histiocytic sarcoma in the lamina propria. (H&E ×400); (d) Immunohistochemistry for CD68 was positive (CD68 ×400).
Figure 2: (a) Gross appearance of cystectomy specimen; (b) Low-power view showing papillary urothelial neoplasm and invasive nests of tumor in lamina propria (H&E, ×200); (c) High-power view showing the focus of histiocytic sarcoma in the lamina propria. (H&E ×400); (d) Immunohistochemistry for CD68 was positive (CD68 ×400).
The cystoscopic examination revealed two suspicious areas in close proximity to one another, each measuring 30 mm with solid-papillary character at the left side-wall-contralateral-wall junction and at the superior aspect of the left ureteral orifice. Based on the clinical findings, tissue samples were collected by means of transurethral resection. Light microscopic evaluation of haematoxylin and eosin (HE)-stained tissue samples revealed neoplastic clusters consisting of stratified urothelial cells with altered cytological structure around a papillary core of irregular appearance, and solid areas in lamina propria. Diffuse sheets of large cells with hyperchromatic nuclei and ample clear cytoplasm, with a patternless pattern among small lymphocytes in a myxoid background, appeared to completely fill the bladder submucosa adjacent to the neoplastic epithelial nests in the lamina propria (Figure 2).
Histomorphological evaluation of the case showed two distinct neoplastic patterns. High-grade papillary urothelial carcinoma, lymphoepithelioma-like variant of urothelial carcinoma, neuroendocrine carcinoma, malignant melanoma, lymphoma and histiocytic neoplasms were considered in the differential diagnosis. Based on relevant immunohistochemical studies, neoplastic cells representing urothelial carcinoma resulted in a positive reaction with GATA3, CK7 and CK20. Atypical cells without pattern formation, which populated the submucosa reacted positively with CD45, CD68 and vimentin, while partial staining was observed with Bcl-2, and no staining was observed with keratin, CK7, CK20, CD138, S100, ALK, HMB45, Melan-A, CD34, SMA, desmin, PSA, CD30, CD15, CD20, CD3, CD5, CD7, CD4, Bcl-6, Pax8, CD10, chromogranin and synaptophysin. EBER staining applied by SISH was negative. No mucin was detected on mucicarmine staining.
According to histomorphological characteristics and results of immunohistochemical studies, this case was diagnosed as histiocytic sarcoma accompanied by invasive urothelial carcinoma. No metastatic focus was detected in preoperative imaging techniques and the radical cystoprostatectomy + urinary diversion + bilateral pelvic lymph node dissection was performed two weeks after the diagnosis (Figure 2). The macroscopic evaluation revealed a red-colour area measuring 40 mm in size with an indented centre localised in the posterior wall of the bladder, adjacent to an area of ballooning towards the cavity. When the bladder wall was serially sectioned, a thickening of 20 mm was observed. Multiple tissue samples were collected from these different areas. The histomorphological examination showed the presence of atypical, large cells with ample clear cytoplasm scattered in lymphoid stroma without forming a pattern, similar to those seen in transurethral resection material and epithelial tumor infiltration forming solid nests extending to muscularis propria below a necrotic surface. The case was reported as histiocytic sarcoma in combination with high-grade invasive urothelial carcinoma. In addition, it was reported as stage 4a due to invasion of the tumor into the prostate tissue. During the postoperative period, a decision was made by the uro‑oncology board upon the opinion of radiation oncology and medical oncology colleagues that the patient should remain under follow-up by the urology clinic. No recurrence or metastasis was seen up to six months post-operatively.
DISCUSSION
Histiocytic sarcomas in extranodal sites are extremely rare malignancies with poor prognosis. Most of the patients are in an advanced clinical stage (stage III/IV) at the time of presentation, with disease progression leading to death in 60-80% of these cases. In clinical terms, a better prognosis may be seen in patients with localised disease and small tumour size.1
The tumor is located in paracortical areas in the lymph node and sinusoids in the liver and spleen. In these locations, a diffuse and non-cohesive growth pattern is observed in histiocytic cells. Histiocytic sarcoma cells have a well-demarcated, oval-round appearance and contain large amount of eosinophilic cytoplasm. Pleomorphism and necrosis are prominent in histiocytic tumors. In addition, mitotic activity can be seen frequently. In cases with soft tissue involvement, margins might be infiltrative. For the diagnosis, at least one histiocytic or histiocyte-associated immunohistochemical marker (CD163, CD68, CD11c, Lysozyme) should be positive. Ki-67 proliferative index is usually between 5% and 50%.8 The differential diagnoses of histiocytic sarcomas are Hodgkin’s lymphoma, myeloid sarcoma, Langerhans cell histiocytosis, B cell lymphomas, anaplastic large cell lymphoma, dendritic cell sarcoma and metastatic carcinomas. In order to differentiate from these diseases, Cytokeratin (CK), HMB45, Melan-A, MPO, S100, pax-5, CD30, CD15, CD20, CD3, ALK and CD138 stains should be used.
In the literature, BRAF V600E mutation has been reported in the pathogenesis of histiocytic sarcoma, Langerhans cell histiocytosis, follicular dendritic sarcoma and juvenile xanthogranuloma among the histiocytic neoplasms.3
The majority of the available knowledge concerning histiocytic sarcoma is based on the study by Hornick et al., which included 14 patients with unifocal extranodal histiocytic sarcoma. In their case series, surgical resection formed the basis of treatment. The authors suggested that adjuvant radiotherapy may help reduce the rate of local recurrence. They also recommended chemotherapy in case of extensive disease, highlighting the lack of information about the role of adjuvant therapy in unifocal disease. Although the optimal chemotherapy regimen remains uncertain, patients have been most commonly treated with lymphoma-based treatments such as cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) or CHOP and etoposide.9 Coutermarsh-Ott et al. recommended NF-kB signal inhibition in the treatment of histiocytic sarcoma.10 However, there is no clinical study on this subject matter to date.
For the case presented herein, we focused on lymphoma after ruling out the diagnoses of epithelial and neuroendocrine neoplasms and malignant melanoma in areas that exhibited a pattern different from invasive urothelial carcinoma. However, since no B/T immunophenotype was shown with the immunohistochemical findings, we diverted our attention to histiocytic neoplasms. Coexistence of histiocytic sarcoma and urothelial carcinoma was challenging for the diagnosis. Without immunohistochemical findings, the diagnosis of histiocytic sarcoma might have been missed and only invasive urothelial carcinoma might have been diagnosed. We report this case of invasive urothelial carcinoma, which is fairly rare in the bladder, accompanied by histiocytic sarcoma to share our challenging experience and emphasise the necessity to consider haematological malignancies in this setting.
PATIENT’S CONSENT:
The patient’s informed consent was obtained for the publication of this case report.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
AIA: Concept and design of the study, first draft of the manuscript, final approval of the version to be published.
SK: Literature review, manuscript writing.
MA: Proofreading, manuscript writing.
REFERENCES
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th Edition. Lyon 2017.
- Berkmen F, Celebioglu AS. Adult genitourinary sarcomas: A report of seventeen cases and review of the literature. J Exp Clin Cancer Res 1997; 16(1):45-8.
- Go H, Jeon YK, Huh J, Choi SJ, Choi YD, Cha HJ, et al. Frequent detection of BRAF (V600E) mutations in histiocytic and dendritic cell neoplasms. Histopathology 2014; 65(2):261-72. doi: 10.1111/his.12416.
- Philippe ES, Wassim K, Jordan RS, Tomasz T, Mike H, Rita FT, et al. Review of the M.D. Anderson experience in the treatment of bladder sarcoma. Urologic Oncology 2007; 25(1):38-45. doi: 10.1016/j.urolonc.2006.02.003.
- M-Jesus FA, Pablo PA, Cristina DA. Histiocytic sarcoma with bladder involvement: Case report and literature review. Rev Esp Patol 2018; 51(1):23-6. doi: 10.1016/j. patol.2017.01.001.
- Dervisis NG, Kiupel M, Qin Q, Cesario L. Clinical prognostic factors in canine histiocytic sarcoma. Vet Comp Oncol 2017; 15(4):1171-80. doi: 10.1111/vco.12252
- Moore PF. A review of histiocytic diseases of dogs and cats. Vet Pathol 2014: 51(1): 167-84. doi: 10.1177/03009 858135 10413.
- Skala SL, Lucas DR, Dewar R. Histiocytic sarcoma: Review, discussion of transformation from B-cell lymphoma, and differential diagnosis. Arch Pathol Lab Med 2018; 142(11): 1322-9. doi: 10.5858/arpa.2018-0220-RA.
- Hornick JL, Jaffe ES, Fletcher CD. Extranodal histiocytic sarcoma: Clinicopathologic analysis of 14 cases of a rare epithelioid malignancy. Am J Surg Pathol 2004; 28(9): 1133-44. doi: 10.1097/01.pas.0000131541.95394.23.
- Coutermarsh-Ott S, Simmons A, Capria V, LeRoith T, Wilson JE, Heid B, et al. NLRX1 suppresses tumorigenesis and attenuates histiocytic sarcoma through the negative regulation of NF-B signaling. Oncotarget 2016; 7(22): 33096-110. doi: 10.18632/oncotarget.8861.