PET-CT and MR Imaging in the Management of Axillary Nodes in Early Stage Breast Cancer
By Mehmet Tarık Baran1, Hasan Gundogdu2, Gokhan Demiral1, Osman Kupik3, Medeni Arpa4, Ahmet Pergel1Affiliations
doi: 10.29271/jcpsp.2020.09.946ABSTRACT
Objective: To discriminate between malignant or benign axillary lymph nodes in breast cancer using MRI, PET-CT, and sentinel lymph node biopsy.
Study Design: Observational study.
Place and Duration of Study: Department of General Surgery, Recep Tayyip Erdogan University School of Medicine, from January 2014 to March 2019.
Methodology: Sentinel lymph node biopsy (SLNB) or axillary lymph node dissection (ALND) was carried out on 102 patients, who had locally advanced cases and had not previously received neoadjuvant therapy. Axillary lymph nodes pathology results were evaluated and compared with PET-CT and MRI findings.
Results: PET-CT specificity was 93.18%, MRI specificity was 93.75%, and combined PET-CT and MRI specificity was 97.67%. PET-CT sensitivity was 81.03%, MRI sensitivity was 68.57%, and combined PET-CT and MRI sensitivity was 83.05%. For detecting the presence of axillary lymph node metastasis, there was a good correlation between histopathological results and the combined evaluation with PET-CT and MRI (kappa: 0.785, p <0.001). In combined PET-CT and MRI, short diamater mean values of lymph nodes in 10 patients, which could not detect lymph node metastases, were determined to be 5.2 ±0.9 mm.
Conclusion: Combining PET-CT and MRI is superior to PET-CT or MRI imaging alone in distinguishing benign and malignant axillary lymph node; and contributes to deciding the approach to axillary lymph node surgery. Lymph node size is also important for this imaging method to determine benign and malignant nodes correctly.
Key Words: Breast cancer, PET-CT, MRI, Sentinel lymph node biopsy, Axilla.
INTRODUCTION
Breast cancer is the second most frequently diagnosed malignancy in the world after lung cancer; and more than two million new cases are seen each year.1 It is the leading cause of death in women around the world.2
The most important factors determining the prognosis are: tumour size, histological grade, hormonal receptor status, human epidermal growth factor receptor 2 (HER-2) status, axillary lymph node involvement, and metastasis.
Axillary lymph node involvement is very important in terms of prognosis, staging, and treatment planning to be selected for the management of the disease.
The examination to be performed after staging, the treatment modalities to be applied, and the management of the patients were determined in detail by National Comprehensive Cancer Network (NCCN).3
Surgeries are performed specifically for the mass in the breast and for the axilla, depending on the condition of the axillary lymph nodes. Axillary lymph node dissection (ALND) or sentinel lymph node biopsy (SLNB) is performed according to the condition of the axilla. In patients undergoing sentinel lymph node sampling during surgery, it is to be determined whether or not there will be an intervention to the axilla.4 Serious morbid complications, such as lymphedema of the arm, may occur after surgery or local radiotherapy to the axilla.5 In order to establish a good preoperative decision, the objective of this study was to discriminate between malignant or benign axillary lymph nodes in breast cancer using MRI, PET-CT, and sentinel lymph node biopsy.
METHODOLOGY
A total of 102 patients, diagnosed with locally advanced breast cancer between March 2014 and January 2019 at General Surgery Clinic, Recep Tayyip Erdogan University School of Medicine, were retrospectively evaluated after the approval by the Clinical Research Ethics Committee. Those patients were included in the study, who were newly diagnosed, underwent 3-Tesla MRI as well as 18F FDG labelled PET-CT, did not receive neoadjuvant therapy, and had undergone SLNB and/or ALND. Exclusion criteria were patients with metastasis, who had PET-CT and MRI performed at the external centre, under the age of 18 years, and with distortion or artefacts on MRI scan.
PET-CT and MRI were retrospectively reassessed by radiology specialist and nuclear medicine specialist, respectively. Clinical information, pathology results, previous imaging reports, and surgical findings were not shared with them.
MRI was performed on 3-Tesla MRI (Discovery w750, GE Healthcare, United States). Contrast agent was injected intravenously following pre-contrast images. Then dynamic imaging was performed. Diffusion-weighted images were taken without contrast, and the axial plane was obtained by applying a gradient sensitising diffusion at different b values (50 sec / mm², 800 sec / mm²) in three directions (x, y, z).
Tumour characteristics, including the largest dimension, shape, location, and contrast enhancement of primary tumour, were evaluated. To distinguish positive and negative axillary lymph nodes, the diameter of the short axillary lymph node (LnD), short /long axis ratio, asymmetric cortex, indeterminate margins, absence of fatty hilum perinodular oedema, cortical thickness increase, diffusion-weighted imaging, and visual examination of ADC were performed with ADC values (Figures 1a, 1b). Images were uploaded on PACS (picture archiving and communication system).
PET-CT imaging was performed with biograph mCT (Siemens Healthcare, Erlangen, Germany) scanner. Siemens Healthineers Syngo via VB30 workstation, MM oncology and post-processing unit was used in the analysis. The short-axis diameter of the axillary lymph nodes was measured. Maximum standard uptake value (SUVmax) was measured from the most prominent FDG avid lymph node (Figure 1c). Blood pool SUVmax was measured from the aortic arch. As axillary lymph node metastasis criterion, the SUVmax value measured from the lymph node was considered to be higher than the blood pool SUVmax value measured from the aorta.
The mean gap between MRI and axillary surgery was 11 (2: 29) days; and 09 (3: 22) days for PET-CT.
Periareolar region was stained with isosulfan blue, massaged for 15 minutes. Then, the stained first lymph node with the axillary incision was excised and sent to pathology to make for the frozen sentinel section. Sentinel lymph node sampling with isosulfan blue is a method preferred at the study centre because it is easily accessible, cheaper, and its sensitivity is higher than radioisotope sentinel sampling in some studies.6 Although isosulfan blue and radioisotope sentinel lymph node sampling together gave more surgical confidence, in this study, isosulfan blue alone was the preferred method.
Research data were uploaded and evaluated via SPSS (Statistical Package for Social Sciences) for Windows 20.0 (SPSS Inc, Chicago, IL). Descriptive statistics were presented as mean ± SD frequency distribution, and percentage. Diagnosis performances of MRI and PET-CT were evaluated by accepting the pathology as the gold standard. Sensitivity, specificity, PPV, NPV and accuracy rates were determined. The coherence between MRI, 18F-FDG PET-CT, and pathology was evaluated by kappa analysis. Significance level was accepted as p<0.05.
RESULTS
A total of 102 patients, histopathologically diagnosed with breast cancer, were scanned with 18-F FDG PET-CT and MRI before treatment. Their average age was 55.4 ± 12.78 years. Fifty-three (52%) of the patients had cancer in their left breast, and 49 (48%) had the cancer in the right breast. Eighty-eight (86.3%) of all these patients were luminal; 6 (5.9%) were HER-2 positive, and 8 (7.8%) were triple negative. ALND was performed in 70 (68.6%) patients included in the study. Of these patients, 46 (45.1%) patients with clinically and radiologically positive axilla directly had axillary dissection. SLNB was performed in 56 (54.9%) patients, who were clinically and radiologically negative. Depending on their state, axillary dissection with SLNB was performed to 24 (23.5%) of these patients. The sentinel lymph node was not stained with isosulfan blue in 9 (8.8%) patients who underwent ALND; lymph node metastasis was not detected on pathology. Two patients had lymph node metastasis and one patient was negative after ALND pathology results. MRI and PET-CT results were negative in 2 (2.0%) of the patients who were found to be positive. The axillary intervention and sentinel results are given in detail in Table I.
There were 3 (2.9%) patients who had negative sentinel lymph nodes, but underwent axillary dissection due to non-sentinel palpable lymph nodes. As a result of ALND, axilla positive in two patients and negative pathology in one patient. PET-CT was positive in one and negative in one of two axilla-metastatic patients. MRI reported all three patients as negative.
Axillary dissection was performed in all 9 (8.8%) patients with a positive sentinel lymph node. When the MRI and PET-CTs of these patients were evaluated retrospectively, PET-CT was considered to be positive in three patients; whereas, in MRI, axilla was positive in one patient and negative in others. In the pathology report of three of nine SLNB positive patients, metastasis was detected only in one lymph node.
The result of axilla pathology was positive in 53 (52%) patients and negative in 49 (48%) patients. Histopathologically, 52 patients had metastases; and PET-CT could not detect axillary lymph nodes in 11 (21.2%) patients. Histopathologically, 50 patients, who did not have metastases, did not have PET-CT axillary lymph node metastasis in 47 (94%, kappa 0.726, p<0.001). The data are shown in detail in Table II.
Histopathologically, 52 patients had metastases, and MRI could not detect axillary lymph node in 22 (42.3%) patients, kappa 0.533, p<0.001).
Table I: Classification of patients undergoing SLNB and ALND (n: 102).|
Axillary interventions |
Sentinel negative |
Sentinel positive |
Sentinel suspicious |
Sentinel lymph node not found |
|
SLNB+ALND |
3 (2.9%)* |
9 (8.8%) |
3 (2.9%) |
9 (8.8%) |
|
SLNB alone |
32 (31.4%) |
|||
|
ALND alone |
46 (45.1%) |
|||
|
*ALND was performed because there was non-sentinel (+) and malignant suspicious lymph nodes founded. |
||||
Table II: Cross-tabulation of 18F- FDG PET-CT and pathology result (n: 102).
|
Pathological axilla status |
PET-CT no axillary metastasis |
PET-CT axillary metastasis present |
Total |
|
No axillary metastasis |
47 (94%) |
3 (6%) |
50 (100%) |
|
Axilla metastasis present |
11 (21.2%) |
41(78.8%) |
52 (100%) |
|
Total |
58 (56.9%) |
44 (43.1%) |
102 (100%) |
Table III: Cross-tabulation of MRI and pathology result (n: 102).
|
Pathological axilla status |
MR no axillary metastasis |
MR axillary metastasis present |
Total |
|
No axillary metastasis |
48 (96%) |
2 (4%) |
50 (100%) |
|
Axilla metastasis present |
22 (42.3%) |
30 (57.7%) |
52 (100%) |
|
Total |
70 (68.6%) |
32 (31.4%) |
102 (100%) |
Table IV: Sensitivity, specificity, accuracy, positive predictive value, negative predictive value were given by MRI alone, 18F- FDG PET-CT alone, and MRI and 18F-FDG PET-CT together (n: 102).
|
Parameters |
18F- FDG PET-CT |
MRI |
18F- FDG PET-CT + MRI |
|
Sensitivity |
81.03% |
68.57% |
83.05 |
|
Specificity |
93.18% |
93.75% |
97.67% |
|
Positive likehood rate |
11.89 |
10.97 |
35.71 |
|
Negative likehood rate |
0.20 |
0.34 |
0.2 |
|
Positive predictive value |
94% |
96% |
98% |
|
Negative predictive value |
78.85% |
57.69% |
80.77% |
|
Accuracy |
86.27% |
76.47% |
89.22% |
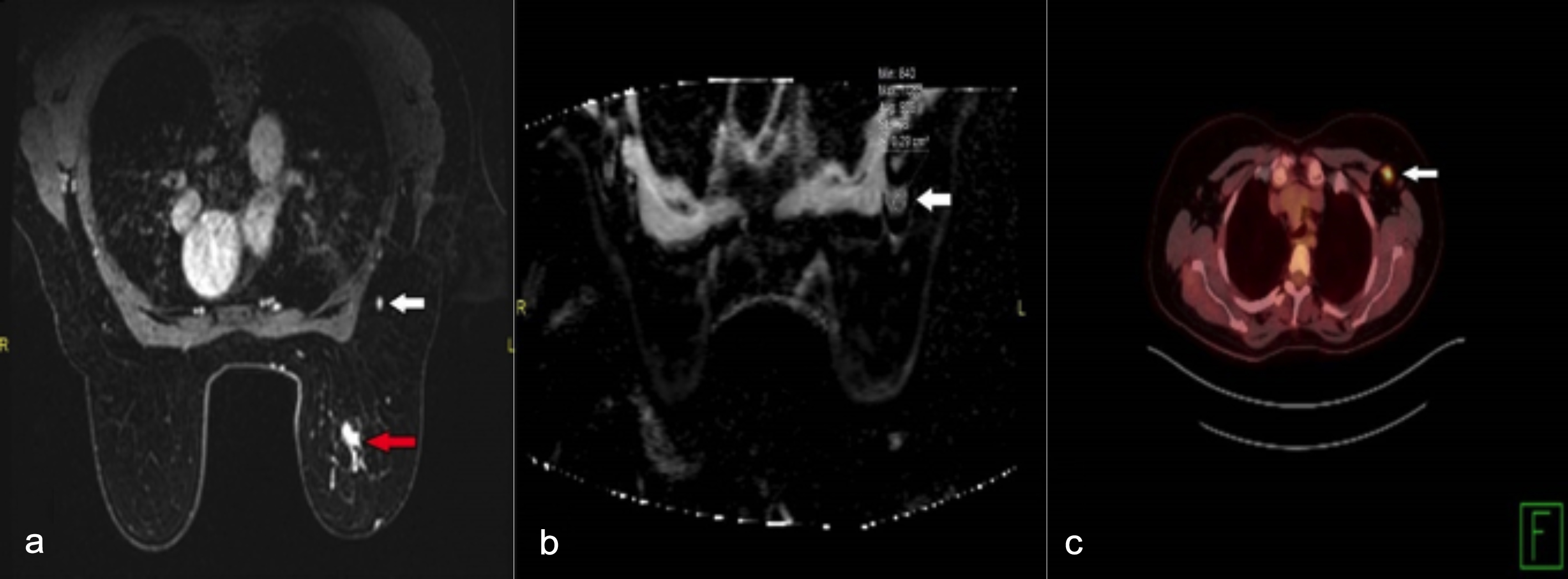 Figure 1: (a) Mass lesion in the left breast with pathological contrast involvement in contrast-enhanced MRI examination (red arrow); and pathological lymph node in the left axilla (white arrow). (b) Diffüsion-weighted MRI of left axillary lymph node (white arrow). The lymph node in Figure 1a has a low ADC value. (c) Significant 18F-FDG uptake of the same lymph node is observed in PET-CT.
Figure 1: (a) Mass lesion in the left breast with pathological contrast involvement in contrast-enhanced MRI examination (red arrow); and pathological lymph node in the left axilla (white arrow). (b) Diffüsion-weighted MRI of left axillary lymph node (white arrow). The lymph node in Figure 1a has a low ADC value. (c) Significant 18F-FDG uptake of the same lymph node is observed in PET-CT.
PET-CT and MRI together detected the presence of metastases in 42 (80.8%) of 52 patients. Forty-nine (98%) of 50 non-metastatic patients were detected (kappa 0.785, p<0.001). A good coherence between the total result of MRI and PET-CT visual evaluation and histopathological results was found. The accuracy and likelihood values are given in Table IV.
When 18F-FDG PET-CT and MRI were evaluated together, the short diameter mean values of lymph nodes PET-CT and MRI were found to be 5.2 mm ± 0.9mm and 6.5 ± 0.85 mm, respectively; in 10 patients, where the presence of lymph node metastases could not be detected.
DISCUSSION
In the management of breast cancer, axillary lymph node involvement is a guide to the prognosis, staging, and treatment planning. Clear determination of the malignant or benign status of the axillary lymph nodes, with the help of radiological imaging, ensures correct staging.7 Successful staging affects prognosis and survival by ensuring the correct treatment plan. In the study by Arriagada et al., four factors were associated with the risk of death. These were tumor size, histological grade, involved axillary lymph node number, and age of diagnosis.8 In this study, PET-CT, MRI, sentinel lymph node sampling, and histopathology methods were used to determine involvement of lymph nodes. In this way, it was aimed to identify the method with less intervention in the axillary region and to show the accuracy of radiological methods to the management of breast cancer surgery.
Apart from screening and diagnosis, imaging is an important guide in clarifying staging.In this context. Breast ultrasound and breast MR are recommended for diagnostic purposes, based on screening mammography by NCCN.There are many different imaging modalities for diagnosis and staging purposes.There are many studies such as PET-CT, MRI, USG or combinations of these for the selection of axilla surgery for axilla.9-13
As in this study, there are few studies that PET-CT and MRI are together in the literature.14 In this study, patients who underwent 18F-FDG PET-CT and MRI to determine the breast lesion were preferred for axillary staging.
In study by McDonough et al., comparing with other similar studies, it was revealed that the number and size of axillary lymph node involvement were important criteria in determining axillary metastasis with PET-CT. Considering all the studies of PET-CT, its sensitivity was in the range of 79% -100%, and specificity was between 66-100%.15 In the present study, PET-CT sensitivity was 81.03%, specificity was 93.18%; and similar results were found with previous studies. Although PET-CT is very successful in axillary staging, some studies have shown that PET-CT cannot replace ALND and SLNB.16
McDonough et al. compared some studies and reported that only one lymph node involvement was observed in 25% of patients with sentinel lymph node micro-metastasis. If an appropriate technique is used to determine micro-metastases, it is thought that the need for sentinel lymph node sampling and axillary dissection may decrease; and the patient will be exposed to less morbid complications.15 In this study, only one postive lymph node involvement was observed in three out of nine cases, 33.3% of which were sentinel lymph node positive. According to the ACOSOG Z0011 study, ALND and radiotherapy have been shown to have the same survival results in patients with early-stage clinically axilla negative and SLNB and 1-2 metastatic sentinel lymph nodes. In the future, imaging methods have become more important for unnecessary SLNB.17,18
In the first studies on PET-CT in breast cancer diagnosis and staging, its accuracy has been shown to vary, depending on the size of the tumour and the diameter of the axillary lymph node. It was more difficult to detect positive lymph nodes in lymph nodes less than 1 cm in diameter. Lesions that are not palpable and cannot be clearly identified by other imaging modalities are detected by PET-CT.19 Similarly, PET-CT and MRI accuracy rate was found to be increased according to the lymph node diameter in this study.
Kvistad et al. have been shown to be able to display lymph nodes better with dynamic MRI, but the number of false negatives was not negligible. Sensitivity was 83%, and specificity was 90%.20 Comparing with our study, in this study the sensitivity in evaluating the axillary lymph nodes was found to be 68.57%, and the specificity was 93.75%.
In the study by An et al., the sensitivity of MRI alone was found to be 67.5%, and the specificity was 78%. The sensitivity of PET-CT alone was 62.7%, and the specificity was 88.6%. The sensitivity of the association of both imaging methods was 72.3% and the specificity was 92.4%. In the diagnosis and evaluation of axillary lymph nodes, it was seen that the evaluation of imaging modalities together increased accuracy rates, sensitivity, specificity, and predictive value.9 Overlapping with this work, the sensitivity and specificity of PET-CT and MRI alone were lower than the sensitivity and specificity with which they were evaluated rather than evaluating alone. Evaluating both the imaging methods together gave clearer and more accurate results.
The main limitations of this study was a single centre, and retrospective analysis. The authors believe that with the development of imaging methods and wider study results, axillary intervention rates will decrease. When PET-CT and MRI are used together, the contribution of the axilla to the surgical management will become clearer with the studies with more participation in the future.
CONCLUSION
In axillary management, evaluating with both 18F-FDG PET-CT and MRI together yielded higher sensitivity and specificity than evaluating them alone in patients with early-stage breast cancer. Compared with ALND and SLNB, it suggests that these imaging methods can be non-invasive complementary methods with their diagnostic performance.18-FDG PET-CT and MRI are good diagnostic methods for surgical selection in the approach to axilla; but both imaging methods cannot replace SLNB in making ALND decisions in axilla clinically negative patients.
ETHICAL APPROVAL:
This study was conducted at General Surgery Clinic, Recep Tayyip Erdogan University School of Medicine after the approval by the Clinical Research Ethics Committee.
PATIENTS' CONSENT:
Informed consents were obtained from all the patients prior conducting the study.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS' CONTRIBUTION:
MTB, AP, GD: Clinical investigators, served as scientific advisors.
MTB, OK, HG: Critically reviewed the study proposals, collected data, provided and cared for study patients.
MTB, AP, MA: Participated in writing or technical editing of the manuscript.
REFERENCES
- The Global Cancer Observatory G. Breast Cancer. Source: Globocan 2018. World Heal Organ [Internet]. 2018; 876:2018–9. Available from: http://gco.iarc.fr/today
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69(1):7-34. doi: 10.3322/caac.21551.
- Telli ML, Gradishar WJ, Ward JH. NCCN guidelines updates: Breast cancer. J Natl Compr Canc Netw 2019; doi: 10.6004/ jnccn.2019.5006.
- Jones C, Lancaster R. Evolution of operative technique for mastectomy. Surg Clin North Am 2018; 98(4):835-44. doi.org/10.1016/j.suc.2018.04.003
- Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol 2010; 102(2):111–8. doi: 10.1002/jso.21535
- Donahue EJ. Sentinel node imaging and biopsy in breast cancer patients. Am J Surg 2001; 182(4):426–8. doi.org/ 10.1016/S0002-9610(01)00731-0
- Valente SA, Levine GM, Silverstein MJ, Rayhanabad JA, Weng-Grumley JG, Ji L, et al. Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol 2012; 19(6):1825–30. doi: 10.1245/s10434- 011-2200-7.
- Arriagada R, Le MG, Dunant A, Tubiana M, Contesso G. Twenty-five years of follow-up in patients with operable breast carcinoma: Correlation between clinicopathologic factors and the risk of death in each 5-year period. Cancer 2006; 106(4):743–50. doi: 10.1002/cncr.21659.
- An YS, Lee DH, Yoon JK, Lee SJ, Kim TH, Kang DK, et al. Diagnostic performance of 18F-FDG PET/CT, ultra-sonography and MRI. Nuklearmedizin 2014; 53(03):89–94. doi: 10.3413/Nukmed-0605-13-06
- Ergul N, Kadioglu H, Yildiz S, Yucel SB, Gucin Z, Erdogan EB, et al. Assessment of multifocality and axillary nodal involvement in early-stage breast cancer patients using 18F-FDG PET/CT compared to contrast-enhanced and diffusion- weighted magnetic resonance imaging and sentinel node biopsy. Acta radiol 2015; 56(8):917-23. doi: 10.1177/ 0284185114539786.
- Panda SK, Goel A, Nayak V, Shaik Basha S, Pande PK, Kumar K. Can preoperative ultrasonography and mrı replace sentinel lymph node biopsy in management of axilla in early breast cancer — A Prospective study from a tertiary cancer center. Indian J Surg Oncol 2019; 10(3):483-8. doi: 10.1007/s13193-019-00924-7.
- Ueda S, Tsuda H, Asakawa H, Omata J, Fukatsu K, Kondo N, et al. Utility of 18F-fluoro-deoxyglucose emission tomo-graphy/ computed tomography fusion imaging (18F-FDG PET/CT) in combination with ultrasonography for axillary staging in primary breast cancer. BMC Cancer 2008; 8:1–10. doi: 10.1186/1471-2407-8-165.
- Grueneisen J, Nagarajah J, Buchbender C, Hoffmann O, Schaarschmidt BM, Poeppel T, et al. Positron emission tomography/magnetic resonance ımaging for local tumor staging in patients with primary breast cancer: A comparison with positron emission tomography/computed tomography and magnetic resonance ımaging. Invest Radiol 2015; 50(8):505-13. doi: 10.1097/RLI. 0000000000000197.
- Liang X, Yu J, Wen B, Xie J, Cai Q, Yang Q. MRI and FDG-PET/CT based assessment of axillary lymph node metastasis in early breast cancer: A meta-analysis. Clin Radiol 2017; 72(4):295–301. doi: 10.1016/j.crad. 2016. 12.001.
- McDonough MD, DePeri ER, Mincey BA. The role of positron emission tomographic imaging in breast cancer. Curr Oncol Rep 2004; 6(1):62–8.
- Lovrics PJ, Chen V, Coates G, Cornacchi SD, Goldsmith CH, Law C, et al. A prospective evaluation of positron emission tomography scanning, sentinel lymph node biopsy, and standard axillary dissection for axillary staging in patients with early stage breast cancer. Ann Surg Oncol 2004; 11(9):846–53. doi: 10.1245/ASO.2004.11.033
- Giuliano AE, Ballman K V., McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: The ACOSOG Z0011 (Alliance) randomizsed clinical trial. JAMA - J Am Med Assoc 2017; 318(10):918-26. doi: 10. 1001/jama.2017.11470.
- Liberale V, Rosso R, Arisio R, D’Alonzo M, Villasco A, Fuso L, et al. Axillary dissection in patients with preoperative positive nodal cytology: Genuine need or overtreatment? Breast J 2020; 26(2):168–75. doi: 10.1111/tbj.13479.
- Nieweg OE, Kim EE, Wong WH, Broussard WF, Singletary SE, Hortobagyi GN, et al. Positron emission tomography with fluorine‐18‐deoxyglucose in the detection and staging of breast cancer. Cancer 1993; doi: 10.1002/1097-0142 (19930615)71:12<3920::aid-cncr2820711220>3.0.co;2-n.
- Kvistad KA, Rydland J, Smethurst HB, Lundgren S, Fjøsne HE, Haraldseth O. Axillary lymph node metastases in breast cancer: Preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol 2000; 10(9):1464–71. doi: 10.1007/s003300000370.