Epithelioid Inflammatory Myofibroblastic Sarcoma with Leukemoid Reaction
By Yuan-Yuan Wan1, Cheng-Li Miao2, Shi-Bo Liu2, Tong Zhang3, Cheng-Hua Luo2Affiliations
doi: 10.29271/jcpsp.2022.09.1212ABSTRACT
Epithelioid inflammatory myofibroblastic sarcoma (EIMS) is a rare and aggressive inflammatory myofibroblastic tumour (IMT) variant. This report identifies the first case of EIMS with leukemoid reaction. This is also the first case in which pancreatic infiltration occurred from the disease onset. A 14-year male patient presented with an 18×18×10 cm mass at the retroperitoneal space and a white blood cell (WBC) count of 85×109/L. The mass and the invaded tissues were surgically removed with tumour-free margins. Histopathology and bone marrow aspiration confirmed the diagnosis of EIMS with leukemoid reaction. The tumour recurred with hepatic and pulmonary metastasis one month after the surgery. WBC count also increased progressively with the tumour recurrence. There is no consensus on the treatment of EIMS. Since ALK rearrangement presents in all the EIMS cases, surgical resection combined with crizotinib or other targeted drugs may improve the prognosis.
Key Words: Sarcoma, Soft tissue neoplasms, Leukemoid reaction, Crizotinib.
INTRODUCTION
The inflammatory myofibroblastic tumour (IMT) is an intermediate-grade mesenchymal neoplasm that rarely metastasises.1 Prognosis is often favorable with complete resection.2 Epithelioid inflammatory myofibroblastic sarcoma (EIMS) was first proposed by Mariño-Enríquez et al. in 2011, and it is thought to be a rare and aggressive IMT variant.3 Leukemoid reaction (LR) is defined as a white blood cell (WBC) count of >50×109/L that is associated with the malignancy, severe infection, severe hemorrhage, and so on. LR can only be diagnosed after myeloproliferative disorders, and bone marrow (BM) metastases of tumours have been ruled out.4 To the existing knowledge, this is the first reported case of EIMS with LR.
CASE REPORT
A previously healthy 14-year male patient presented to a local hospital with severe abdominal pain in October 2019. CT scan indicated a cystic solid mass located in the left retroperitoneal space, extensive hemoperitoneum and effusion in the abdominal and pelvic cavities, and splenomegaly.
The abdominal pain was relieved by the interventional embolisation and blood transfusion, but a progressive increase in the size of the mass was detected by CT. The patient was then transferred to our Retroperitoneal Tumours Center at Peking University International Hospital (Beijing, China) in December 2019. A CT scan revealed an 18×18×10 cm mass located in the retroperitoneal space that had adhered to the nearby tissues and surrounded the pancreatic tail and splenomegaly (Figure 1). Laboratory results revealed a WBC count of 85×109/L (Figure 2), 91% neutrophils, and normal hemoglobin and platelets. Marked mature neutrophilia with a left shift was detected on the peripheral blood smear. After admission, the patient developed symptoms of intestinal obstruction such as severe vomiting after eating, obvious abdominal distension, and abdominal pain. Then the mass, pancreatic body and tail, spleen, partial transverse, descending colon, corresponding mesentery, and omentum were surgically removed with no visible remnants. The intraoperative blood loss was about 5 L. After blood transfusion and hemostatic treatment, the bleeding stopped and the patient's condition stabilised.
The WBC count returned to 16×109/L after surgery but rose to 41×109/L four days later. Bone marrow (BM) aspiration and biopsy were performed, which showed normal cellularity with myeloid hyperplasia, intact maturation, and morphology. BM immuno-phenotyping showed a mature granulocyte pattern, cytogenetics showed 46, XY, and molecular analysis revealed negative myeloproliferative neoplasm mutations and fusion genes (e.g. BCR-ABL). Histological examination of the retroperitoneal mass showed most of the tumour cells were epithelioid-to-round with vesicular chromatin, large nucleoli, and mitosis around 10–30/10 HPF. About 20% of the tumour cells were spindle-shaped (Figure 3). Prominent neutrophilic inflammatory infiltration and large areas of necrosis were seen. The tumour extensively invaded the pancreas, adipose tissue of the splenic hilum, and mesentery of the colon. The margins were free of tumours. Immunohistochemistry indicated ALK-positive cytoplasm staining with perinuclear accentuation (Figure 4), vimentin +, desmin-, SMA-, CK-, CD30 (scattered +), myogenin -, TP53 +, Ki-67 (70 % +), calponin -, and S-100. FISH showed ALK rearrangement. These findings were consistent with the diagnosis of EIMS and LR.
The patient's condition was stable during the first week after surgery; however, about ten days after the surgery, pancreatic fistula, abdominal bleeding, fever, and colonic anastomotic fistula appeared successively. The patient received treatments including a blood transfusion, hemostasis, analgesia, antibiotics, intravenous nutrition, hydration, and alkalisation. Chemotherapy, targeted drugs, and other combination therapy were not given due to his unstable condition. The fever quickly improved, and the abdominal bleeding, pancreatic fistula, and colonic anastomotic fistula were gradually controlled. Unfortunately, symptoms of the gastrointestinal obstruction appeared and gradually worsened one month after surgery. CT and angiography revealed recurrence of the retroperitoneal tumour with hepatic and pulmonary metastasis. This suggested that the gastrointestinal obstruction was caused by tumour compression. WBC count also increased progressively with the tumour recurrence. The patient died three months after disease presentation with 183×109/L WBC count.
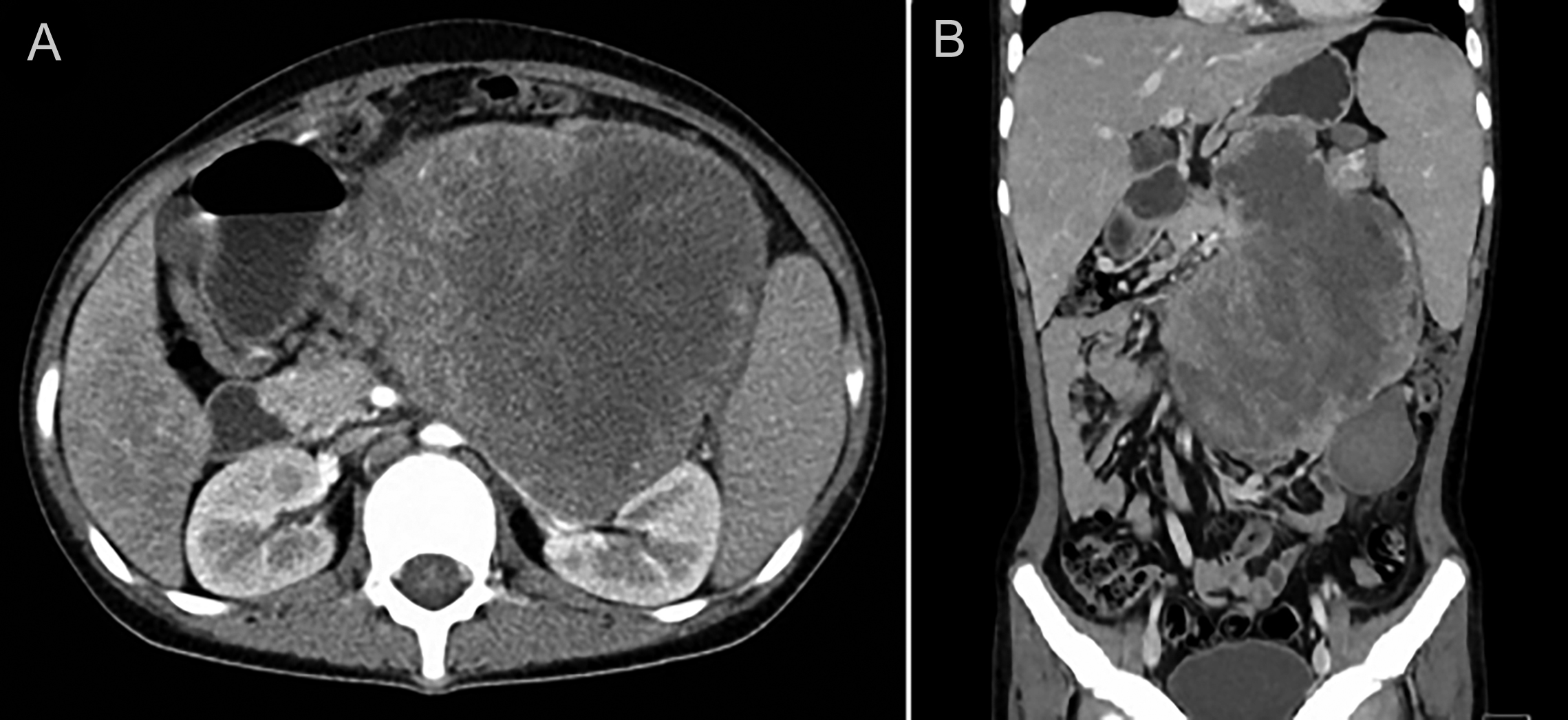 Figure 1: (A, B) Contrast-enhanced CT scan revealing the retroperitoneal tumour.
Figure 1: (A, B) Contrast-enhanced CT scan revealing the retroperitoneal tumour.
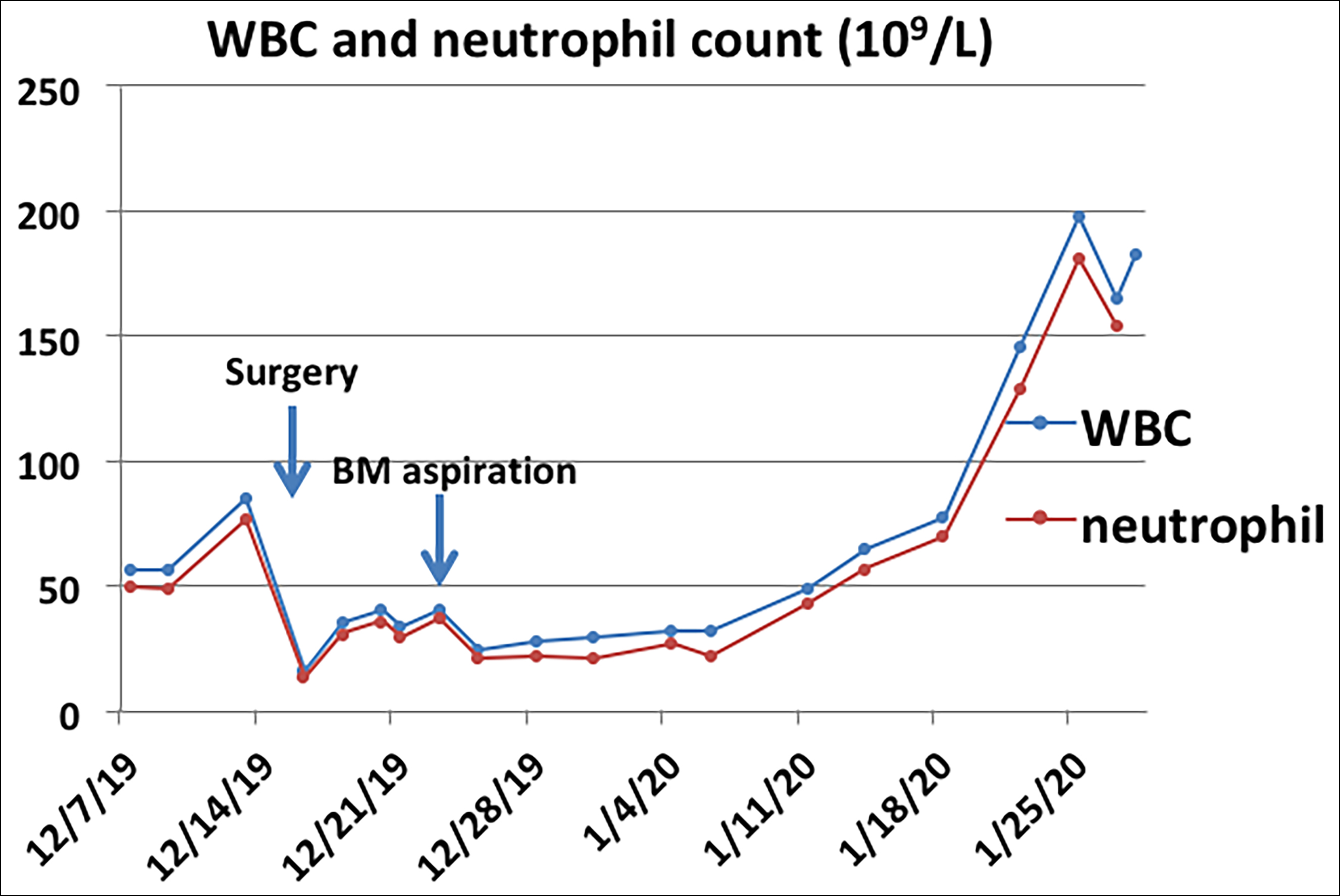 Figure 2: WBC and neutrophil count (peripheral blood) of this case. WBC = white blood cell.
Figure 2: WBC and neutrophil count (peripheral blood) of this case. WBC = white blood cell.
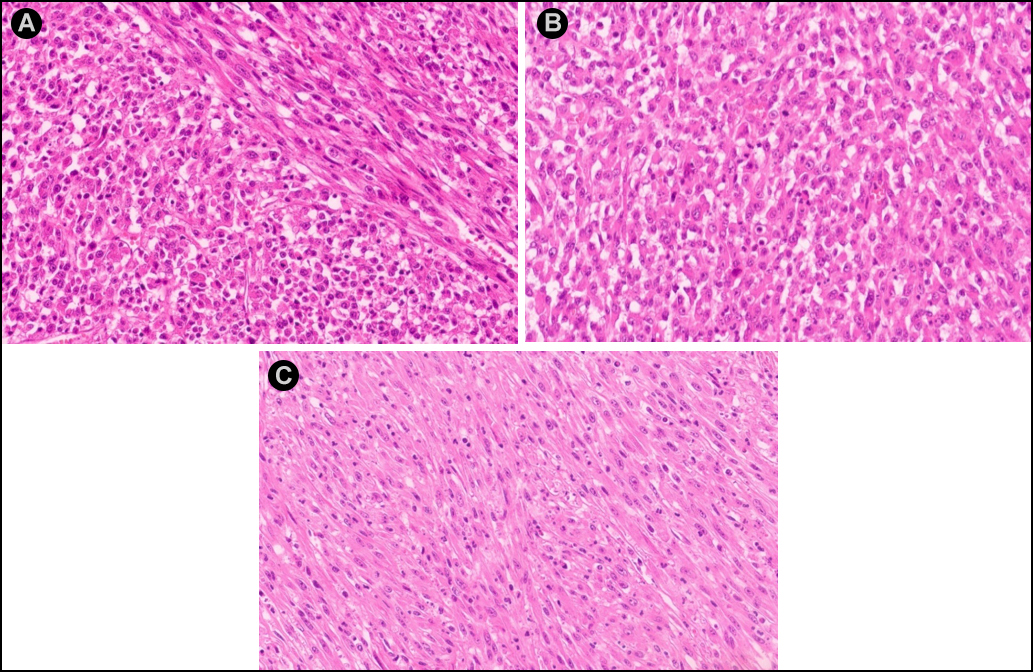 Figure 3: (A) H&E staining showing most tumour cells to be epithelioid-to-round shaped and only a minor portion of spindly cells. (B) Epithelioid-to-round cells. (C) Spindle cells. ( H&E, ×200).
Figure 3: (A) H&E staining showing most tumour cells to be epithelioid-to-round shaped and only a minor portion of spindly cells. (B) Epithelioid-to-round cells. (C) Spindle cells. ( H&E, ×200).
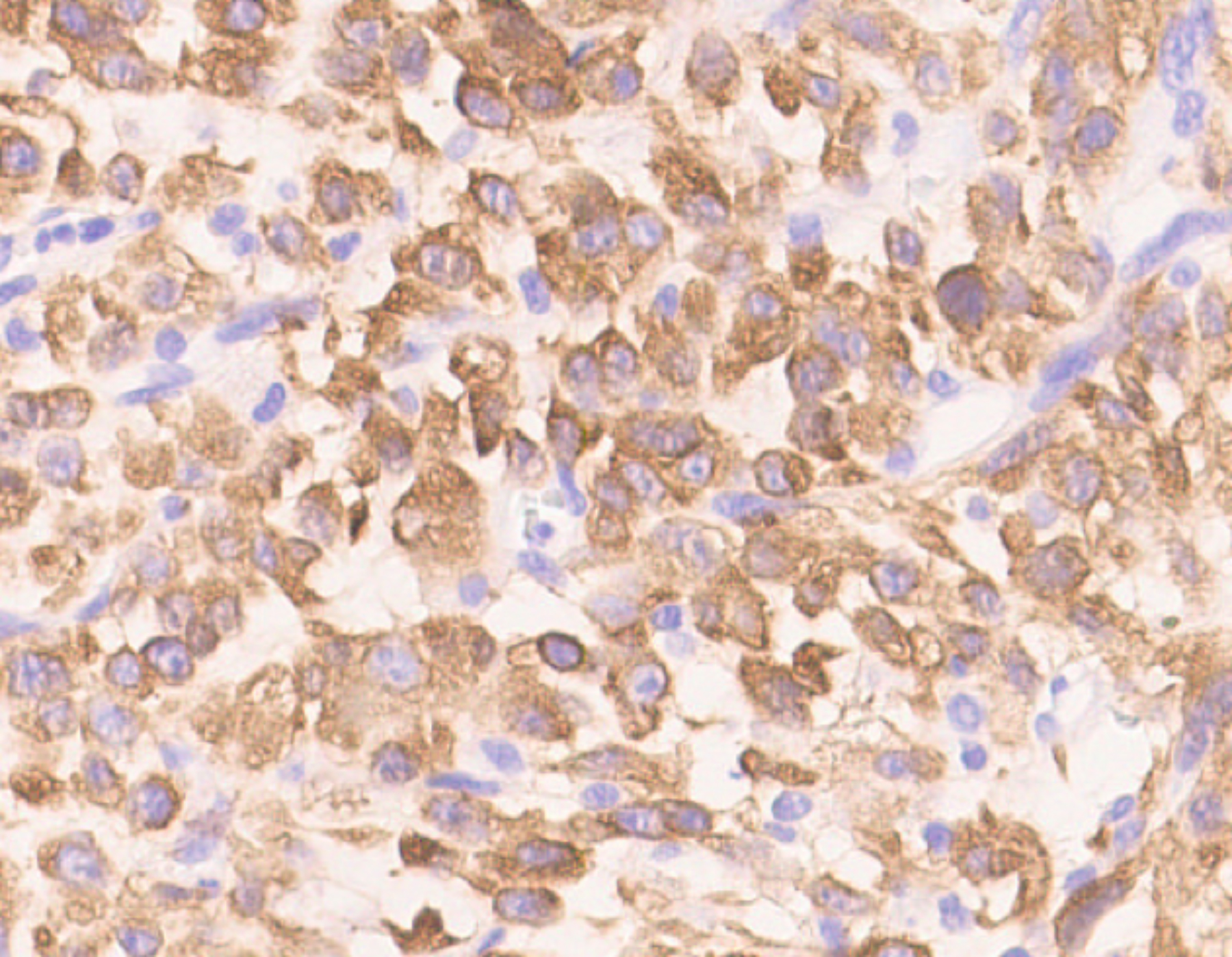 Figure 4: Immunohistochemistry showing ALK-positive cytoplasm with perinuclear accentuation.
Figure 4: Immunohistochemistry showing ALK-positive cytoplasm with perinuclear accentuation.
DISCUSSION
Although EIMS is an IMT variant, its clinical features and prognosis are quite different from a common IMT. EIMS is a highly aggressive tumour and prone to early metastasis. The most common sites of origin for EIMS are the abdominal cavity, mesentery, omentum, and peritoneum. To our knowledge, only one case of EIMS that originated in the spleen has been reported. In addition, only one case of pancreatic head metastasis occurring two years after the disease onset has been reported before. The present case occurred in the retroperitoneum with infiltration of the pancreas, perisplenic tissue, and mesentery of the colon. This is the first case in which pancreatic infiltration occurred at the time of disease onset. Although the lesion was completely resected during surgery, the tumour recurred rapidly with liver and lung metastasis. This confirms the highly malignant characteristic of EIMS.
Early diagnosis is usually difficult due to the rarity of this disease. The differential diagnosis includes ALK-positive anaplastic large cell lymphoma, rhabdomyosarcoma, extranodal follicular dendritic cell sarcoma, and so on. The morphological features of EIMS are that most of the tumour cells are epithelioid-to-round, a small number are spindly with frequent mitoses and abundant myxoid stroma with inflammatory infiltration. The most typical immunohistochemical feature of EIMS is ALK-positive nuclear membrane staining or ALK-positive cytoplasmic staining with perinuclear accentuation.3,4 This case presented with ALK-positive cytoplasmic staining with perinuclear accentuation. All the EIMS cases show ALK rearrangements and most are RANBP2-ALK positive. RRBP1-ALK5 and EML4-ALK6 rearrangements have also been reported.
There is no clear consensus on the treatment of EIMS. Surgical resection is recommended when possible, but recurrence is common. Recent successes of ALK-inhibitors like crizotinib, ceritinib, and alectinib in EIMS/IMT suggest their potential for the disease treatments.7-9 The effectiveness of other combination therapies such as chemotherapy, radiotherapy, non-steroidal anti-inflammatory drugs (NSAIDs), and steroids is uncertain in this rare disease.
Only two cases of IMT with metastasis to BM have been reported in the previous literature.10 WBC counts were significantly elevated in our case, prompting us to consider the possibility of hematologic tumours or BM metastasis of tumours; however, BM examination ruled out those assumptions and revealed the diagnosis of LR. The surgery only removed retroperitoneal tumours, and no chemotherapy was given. If there were hematologic tumours or EIMS tumours in the BM before surgery, those tumours would still be present after surgery. Therefore, the BM examination performed after surgery when the WBC rose again, could represent the status of the BM before surgery. The WBC count rose to nearly 200×109/L before death, but it was uncertain whether there was BM metastasis before the patient died. Paraneoplastic leukemoid reaction (PLR) usually occurs in terminally metastatic tumours, suggesting a poor prognosis. There is a negative correlation between the WBC count at the presentation and the peak WBC count with survival in patients with PLR.11 PLR may also be one of the markers of tumour recurrence. The treatment of LR mainly lies in the treatment of the primary disease. With the removal of the original disease, LR usually improves on its own. For the patients with extremely high WBC counts or severe clinical symptoms, hydroxyurea, hydration, and alkalisation can be added to their treatment to prevent organ embolism caused by the leukocyte stasis.
In summary, EIMS is a highly aggressive IMT variant. We present here the first case of EIMS with LR, as well as the first case in which pancreatic infiltration occurred from the beginning of the disease process. Since ALK rearrangements are found in all the EIMS cases, the combination therapy of surgical resection and crizotinib or other targeted drugs may be the potential treatment. Disease understanding and study of EIMS among pathologists and clinicians need to be strengthened to improve the prognosis of this disease.
PATIENT’S CONSENT:
The authors certify that they have obtained the appropriate patient’s consent form. In the form, the family members of this patient have given consent for his images and other clinical information to be reported in the journal.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
YYW: Data collection, literature search, manuscript writing, and bone marrow aspiration.
CLM, SBL, CHL: Clinical care of the patient.
TZ: Histopathological diagnosis and figures.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Yu L, Liu J, Lao IW, Luo Z, Wang J. Epithelioid inflammatory myofibroblastic sarcoma: A clinicopathological, immuno-histochemical and molecular cytogenetic analysis of five additional cases and review of the literature. Diagn Pathol 2016;11 (1):67. doi: 10.1186/s13000-016-0517-z.
- Dalton BG, Thomas PG, Sharp NE, Manalang MA, Fisher JE, Moir CR, et al. Inflammatory myofibroblastic tumours in children. J Pediatr Surg 2016; 51(4):541-4. doi: 10.1016/j.-jpedsurg.2015.11.015.
- Mariño-Enríquez A, Wang WL, Roy A, Lopez-Terrada D, Lazar AJ, Fletcher CD, et al. Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumour with nuclear membrane or perinuclear ALK. Am J Surg Pathol 2011; 35(1):135-44. doi: 10.1097/PAS.0b013e318200cfd5.
- Portich JP, Faulhaber GAM. Leukemoid reaction: A 21st-century cohort study. Int J Lab Hematol 2020; 42(2):134-9. doi: 10.1111/ijlh.13127.
- Lee JC, Li CF, Huang HY, Zhu MJ, Mariño-Enríquez A, Lee CT, et al. ALK oncoproteins in atypical inflammatory myofibro-blastic tumours: Novel RRBP1-ALK fusions in epithelioid inflammatory myofibroblastic sarcoma. J Pathol 2017; 241 (3):316-23. doi: 10.1002/path.4836.
- Jiang Q, Tong HX, Hou YY, Zhang Y, Li JL, Zhou YH, et al. Identification of EML4-ALK as an alternative fusion gene in epithelioid inflammatory myofibroblastic sarcoma. Orphanet J Rare Dis 2017; 12(1):97. doi: 10.1186/ s13023-017-0647-8.
- Butrynski JE, D'Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, Jhanwar SC, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumour. N Engl J Med 2010; 363(18):1727-33. doi: 10.1056/NEJMoa1007056.
- Theilen TM, Soerensen J, Bochennek K, Becker M, Schwabe D, Rolle U, et al. Crizotinib in ALK+ inflammatory myofibro-blastic tumours - current experience and future perspectives. Pediatr Blood Cancer 2018; 65(4). doi: 10.1002/pbc.26920.
- Casanova M, Brennan B, Alaggio R, Kelsey A, Orbach D, Van Noesel MM, et al. Inflammatory myofibroblastic tumour: The experience of the European pediatric soft tissue sarcoma study group (EpSSG). Eur J Cancer 2020; 127:123-9. doi: 10.1016/j.ejca.2019.12.021.
- Liu Q, Wei J, Liu X, Wang J. Anaplastic lymphoma kinase-negative pulmonary inflammatory myofibroblastic tumour with multiple metastases and its treatment by Apatinib: A case report. Medicine (Baltimore) 2019; 98(52):e18414. doi: 10.1097/MD.0000000000018414.
- Chakraborty S, Keenportz B, Woodward S, Anderson J, Colan D. Paraneoplastic leukemoid reaction in solid tumours. Am J Clin Oncol 2015; 38(3):326-30. doi: 10. 1097/COC.0b013e3182a530dd.