Allium Sativum Oil as an Alternative Non-Vital Pulpotomy Medicament in Primary Teeth - A Randomised Controlled Trial
By Mashal Mazhar1, Shazia Naz2, Ambreen Zahra3, Nazia Bashir4, Muhammad Asdaq Hussain5Affiliations
doi: 10.29271/jcpsp.2024.03.267ABSTRACT
Objective: To use Allium sativum oil as non-vital pulpotomy medicament in primary teeth by evaluating its antibacterial effect (Colony-Forming Units/ml- CFU/ml), against Streptococcus mutans and Lactobacillus acidophilus.
Study Design: A double-blinded, randomised controlled trial.
Place and Duration of the Study: Paediatric Dentistry Department, de’ Montmorency College of Dentistry, Lahore in collaboration with the Microbiology Department, Lahore General Hospital, from October 2022 to February 2023.
Methodology: Forty patients aged between 4 to 8 years, each containing at least one non-vital primary molar, were randomly divided into Group A (Formocresol) and Group B (Allium sativum oil) using the lottery method. Non-vital pulpotomy (NVP) was performed by removing the coronal necrotic pulp. Sterile paper points were dipped in the root canals and taken to the laboratory. Cotton pellets soaked in the respective medicaments were placed over the root canal orifices and filled temporarily. Patients were recalled after one week. Samples were again taken, and the tooth was restored. Comparison was made between bacterial count at baseline and after one week of treatment, and it was expressed as CFU/ml.
Results: There was a significant reduction in median Streptococcus mutans and Lactobacillus acidophilus bacterial count in each group after one week of treatment (p <0.001). Formocresol showed a higher average reduction (30300 ± 14060) compared to Allium sativum oil (24850 ± 9121). However, statistically, the difference was insignificant (p = 0.314) indicating both the medicaments possessed comparable antibacterial effects.
Conclusion: Allium sativum oil was found an effective alternative to Formocresol.
Key Words: Formocresol, Allium sativum, Non-vital pulpotomy, Primary teeth, Randomised controlled trial.
INTRODUCTION
Preservation of cariously exposed primary teeth till their physiological exfoliation is one of the major problems in paediatric dentistry.1 If primary teeth are left untreated, bacterial invasion from the coronal to the radicular pulp and then beyond the root canal into the adjacent soft tissue occurs. This leads to aggressive and painful inflammatory reaction rendering the tooth irreversibly inflamed or non-vital.2 Retention of primary teeth is imperative as their untimely loss can lead to undesirable consequences.3
Bacteria present in primary teeth with necrosed pulp are poly-microbial, predominantly Streptococcus mutans, and Lactobacillus acidophilus.4 S. mutans is commonly regarded as the dental caries initiator,5 and L. acidophilus as dental caries progressor.6
Treatment of choice to save cariously exposed non-vital yet restorable primary teeth is pulpectomy, as the damaged pulp is unable to heal itself.7 Despite its high success rate, pulpectomy is often impractical in some paediatric patients, such as, when there are difficulties in obtaining adequate access due to small mouth openings in children, severe gag-reflex, possible damage to permanent tooth germ, complex (ribbon-shaped) non-negotiable root canals, and limited patient cooperation.1,7 Extraction is another option for the management of non-vital primary tooth, where the tooth is non-restorable due to extensive caries and with severe mobility.8
Non-vital pulpotomy (NVP) also known as Mortal pulpotomy, is regarded as a quick and least invasive alternative with predict-able outcomes for the decayed but restorable primary teeth that are non-vital. The procedure is performed when neither pulpectomy nor extraction is feasible. In NVP, the infected non-vital radicular pulp is dressed with a strong antiseptic material to combat infection, mummify, fix, and sterile the necrotic pulp tissue, so that it can stay in situ till the tooth exfoliates.9
Several conventional materials have been used in pulpotomy, commonly Mineral Trioxide Aggregate (MTA),10 Glutaraldehyde, and Ferric sulfate. However, the ‘gold standard’ material due to its potent antibacterial and fixative properties is Formocresol. Its constituents, formaldehyde and cresol, are responsible for harmful effects11 such as mutagenicity, carcinogenicity, allergenicity, systemic distribution, tissue changes in internal organs, and enamel defects in permanent successors.1
Several herbal medicaments have been used in pulpotomy procedures in paediatric patients because of their wide therapeutic properties, minimum side effects, low cost, easy availability, and long-lasting therapeutic effects with the added benefit of minimum bacterial resistance.12 Garlic (Allium sativum) possesses potent antibacterial potential,12 being anti-inflammatory, analgesic, antioxidant, and anti-cariogenic.13 It is effective against both gram-positive and gram-negative bacteria and has 1000-fold less bacterial resistance than other antibiotics.12 There is literature on A. sativum use as vital pulpotomy medicament,14 but very limited literature as a non-vital pulpotomy medicament.1,7
There is a lack of international literature, and no local study could be found on non-vital pulpotomy procedure and the medicaments used in it. The purpose of the study was to find a suitable herbal alternative (such as Allium sativum oil) to replace the routinely used conventional medicament, Formocresol, in the non-vital pulpotomy procedures.
METHODOLOGY
It was a double blinded, randomised controlled trial, conducted at the Paediatric Dentistry Department, de’ Montmorency College of Dentistry/Punjab Dental Hospital, Lahore in collaboration with the Microbiology Department, Lahore General Hospital, from October 2022 to February 2023. Ethical approval was obtained from the Ethical Review Committee, Post-Graduate Medical Institute/ Ameer-ud-Din Medical College/ Lahore General Hospital, Lahore (AMC/ PGMI/ LGH/ Synopsis No./ 0081-19). The sample size was calculated using WHO Calculator version 12.2.6.
Forty cooperative patients of either gender aged between 4 to 8 years, were selected after the consent of parents, with ASA I and II, no history of antibiotic use for 2 weeks, and having forty non-traumatic, cariously exposed non-vital primary molars (one tooth from each patient), from the Outpatient Department of Paedodontics, de’ Montmorency College of Dentistry, Lahore. Patients with history of allergy to anaesthetics and latex, radiographic evidence of pulpal floor opening into the furcation area, and with more than half of the roots resorbed were excluded.
Participants were enrolled according to their eligibility criteria and randomly allocated into Group A (Formocresol, control group-standard medicament, n=20) and Group B (Allium sativum oil, n=20) using the lottery method. The participants were blinded against which medicament group they belonged. Premade Formocresol (Tricresol and Formalin) was used composed of Tricresol 35%, Formaldehyde (40%) 19%, and excipient additive 100%. Likewise, premade Allium sativum oil (Garlic oil)7 was used.
For ease of procedure, local anaesthesia with 2% lidocaine containing 1:100000 epinephrine was injected, and a rubber dam was applied. Cavity outline and caries removal were done using #330 pear-shaped carbide bur using a high-speed handpiece with constant water spray. Pulp chamber was accessed and de-roofing was done using non-end cutting steel straight fissure bur. Removal of the coronal pulp tissue (if any) was achieved with a sterile, sharp spoon excavator Type-A.7
The samples were collected for in-vitro evaluation. One sample was taken from each root canal by dipping #20 sterile paper point 2-3mm into the root canal for 10 seconds. These paper points were then immediately placed into plastic calibrated screw-capped tubes containing 5ml of autoclaved Fluid Thioglycollate Transport Medium,15 labelled with a laboratory code and transported to the lab as soon as possible.7
The radicular pulp was dressed with #4 (3mm) cotton pellet which was dipped in respective medicament. The tooth was then restored temporarily with a thin layer of Cavit followed by a thick hard mix of Zinc Oxide and Eugenol (ZOE) type II. The patient was then recalled after one week. Again, sample collection was done and transported to the lab. Additionally, the patient was clinically assessed according to standard criteria.7
Blood agar was used for culturing S. mutans, while de Man, Rogosa, Sharpe (MRS) agar was used for L. acidophilus. Culture media plates were prepared according to the manufacturer’s instructions by a laboratory technician. Tubes containing the specimen were vortexed for 60 seconds. One μl portion of the sample was taken with one μl disposable plastic loop and spread on respective culture media plate for quantitative analysis.16 The inoculated plates were then placed in an anaerobic jar with a gas pack and incubated for 2 days (48 hours) at 37°C.7
Bacterial colonies were identified by the naked eye, gram-staining and catalase test. Further identification was done under light microscope with 100X magnification. For species level identification, Analytical Profile Index kit, API 20 STREP kit, and API 50 CHL kit were used for S. mutans and L. acidophilus identification, respectively according to the company’s instructions. The results were entered in licensed API software with database V7.0 for S. mutans and V5.1 for L. acidophilus for interpretation.
To quantify antibacterial effect, S. mutans and L. acidophilus colonies counted with the naked eye were expressed as CFU/ml.
The study was set at 90% power and 95% confidence level with a 5% margin of error. The normality of data was assessed by the Shapiro-Wilk test. Mann-Whitney U test was used for the comparison of bacterial count among different groups. Fisher’s exact test was used to compare clinical outcomes among different groups. A p ≤ 0.05 was considered as significant.
RESULTS
Bacterial colonies of the S. mutans appeared greyish-white, smooth, glossy, and translucent with zones of alpha hemolysis (partial hemolysis with greenish-brown discolouration) on blood agar plates (Figure 1 a,b). They were gram-positive, catalase-negative, and appeared as cocci-shaped, present in pairs and chains, without spores under a light microscope (Figure 1c). Bacterial colonies of L. acidophilus appeared compact, smooth, medium, opaque, and white mucoid on the MRS agar plate (Figure 2 a,b). They were gram-positive, catalase-negative, and appeared as round-ended single, pairs, and chains of rods without spores under a light microscope (Figure 2c). Further biochemical testing using API 20 STREP kit for S. mutans and API 50 CHL kit for L. acidophilus was done, and the results were analysed through API software.
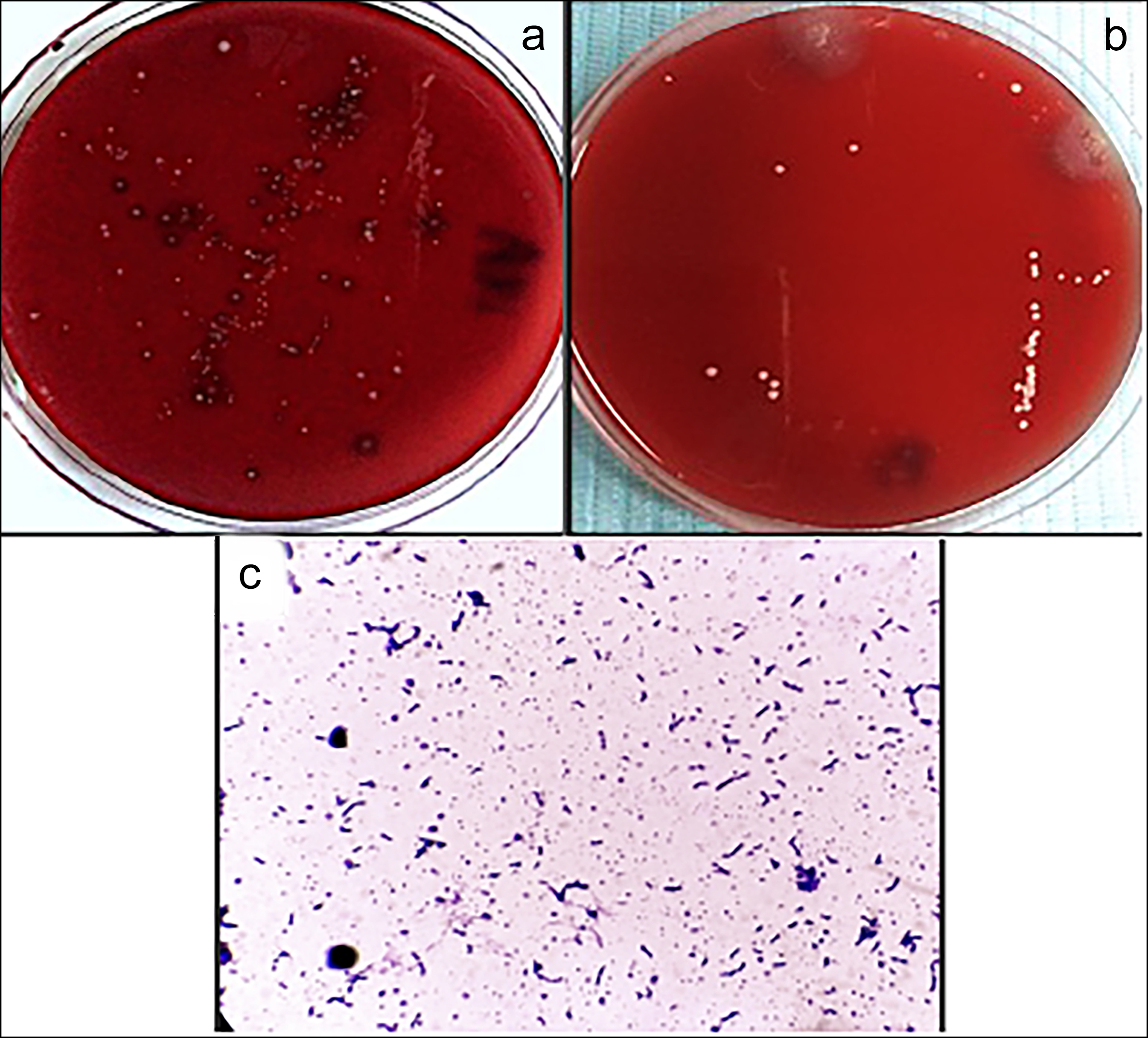 Figure 1: S. mutans colonies on blood agar plate, (a) Polymicrobial cultures at baseline with suspected S. mutans colonies (yellow arrow), (b) Bacterial cultures after one week of medicament placement, (c) Microscopic view of S. mutans.
Figure 1: S. mutans colonies on blood agar plate, (a) Polymicrobial cultures at baseline with suspected S. mutans colonies (yellow arrow), (b) Bacterial cultures after one week of medicament placement, (c) Microscopic view of S. mutans.
The data were not normally distributed according to Shapiro-Wilk test, so median bacterial count for both the groups was calculated. There was statistically no significant difference in the average reduction of S. mutans as well as L. acidophilus bacterial count among the groups according to Mann-Whitney U test. For analysis of combined bacterial count among the groups, the mean bacterial count for both the groups was calculated, as the data were normally distributed. Results of Mann-Whitney U test showed statistically no significant difference in the mean reduction of combined bacterial count among the groups (p = 0.314, Table I) indicating that the overall antibacterial effect of both the medicaments was similar. Clinical outcome was analysed through Fisher’s exact test which revealed that there was statistically insignificant difference in the clinical outcome among the two groups (p >0.999, Table II).
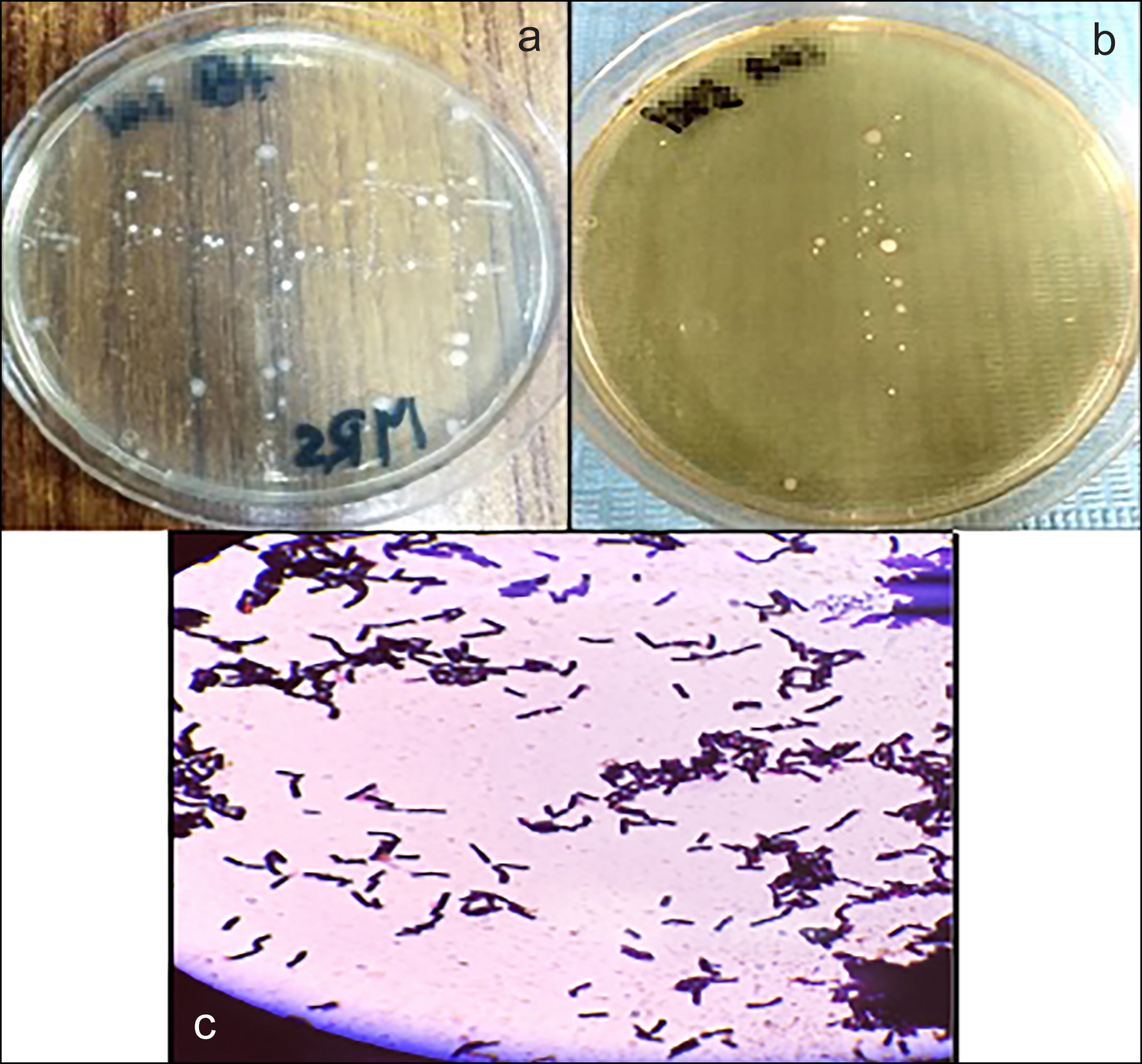 Figure 2: L. acidophilus on MRS agar plate, (a) Polymicrobial cultures at baseline with suspected L. acidophilus colonies (yellow arrow), (b) Bacterial cultures after one week of medicament placement, (c) Microscopic view of L. acidophilus.
Figure 2: L. acidophilus on MRS agar plate, (a) Polymicrobial cultures at baseline with suspected L. acidophilus colonies (yellow arrow), (b) Bacterial cultures after one week of medicament placement, (c) Microscopic view of L. acidophilus.
Table I: Comparison of mean reduction of combined bacterial count among groups.
|
Group |
Mean reduction of overall bacterial count (CFU/ml) |
|||
|
Mean ± SD |
Minimum |
Maximum |
p-value # |
|
|
Group A |
30300 ± 14060 |
13000 |
60000 |
0.314 |
|
Group B |
24850 ± 9121 |
10000 |
43000 |
|
|
Group A: Formocresol, Group B: Allium sativum oil, # Mann-Whitney U test. |
||||
Table II: Comparison between the effects of all the three medicaments on the clinical outcome of non-vital primary teeth after one week (1st follow-up visit).
|
Clinical findings after one week |
Group A [n = 20 (%)] |
Group B [n = 20 (%)] |
p-value* |
|
Postoperative pain |
(+) 2 (10.0%) |
- |
>0.999 |
|
Tenderness to percussion |
- |
(+) 1 (5.0%) |
|
|
Gingival inflammation |
- |
- |
|
|
Draining sinus |
- |
- |
|
|
Increased mobility |
- |
- |
|
|
Group A: Formocresol, Group B: Allium sativum oil, * Fisher’s exact test. + (having signs and symptoms), - (without signs and symptoms). |
|||
DISCUSSION
The study was designed to evaluate the antibacterial effect of Formocresol (Group A), and Allium sativum oil (Group B) against S. mutans and L. acidophilus. These bacteria are the main cause of tooth decay, leading to irreversible damage and, hence, necrosis of tooth. A combined clinical laboratory study was performed to assess the clinical outcome as well of the antibacterial effects of each medicament.
Non-vital (Mortal) pulpotomy procedure was opted in this study as it is often used as a quick, least invasive alternative for decayed, irreversibly inflamed/ non-vital, yet restorable primary teeth. It is performed when neither pulpectomy nor extraction is feasible.9 Pulpectomy is impractical in some paediatric patients with inadequate access, limited cooperation, and possible damage to permanent tooth germ.1,7 Extraction is another option, but it is an invasive procedure and can lead to space maintenance issues.17
The most significant finding of the present study was that both the medicaments showed a significant reduction of S. mutans and L. acidophilus bacterial counts (p <0.001) after one week of treatment. Statistically, there was no significant difference in the individual average reduction (p = 0.820 and 0.277) as well as combined mean reduction (p = 0.314) of S. mutans and L. acidophilus bacterial counts among the groups. This indicated that both the medicaments possessed comparable overall antibacterial effects.
Additionally, in this study, Formocresol exhibited a higher antibacterial effect against S. mutans than L. acidophilus. Allium sativum oil exhibited a higher antibacterial effect against L. acidophilus than S. mutans. This finding adds up to the effectiveness of herbal medicament in irreversibly inflamed/ necrotic primary teeth.
In the present study, the antibacterial effect of Formocresol was strong, especially against S. mutans and L. acidophilus. This finding is concomitant with other studies that have found Formocresol to have strong antibacterial and fixative properties.18,19 Moreover, the overall antibacterial effect of Formocresol was higher compared to Allium sativum oil in the present study. However, statistically, the difference was insignificant, contrary to another similar study in which non-vital pulpotomy procedure was performed in primary non-vital teeth, followed by bacteriological evaluation was done.7
Significant decrease in salivary S. mutans bacterial count was reported by A. sativum extract in a systematic review and meta-analysis as a mouthwash. Although this study was on Allium sativum extract, but resulted in potent antibacterial effect, similar to the present study. The reason might be due to the similar mechanism of action of Garlic. Allicin present in Garlic is responsible for the antibacterial effect by inhibiting thiol-containing enzymes responsible for nutrition of bacteria.20
In the present study, Allium sativum oil showed better clinical outcomes compared to Formocresol as a non-vital pulpotomy medicament in primary molar teeth. However, statistically, the difference was insignificant i.e. p >0.999. Similar to the present study, clinical outcomes of medicaments with statistically insignificant differences have been reported in several other studies in which Formocresol was used as a pulpotomy medicament compared with Allium sativum extract,21 Allium sativum oil,22 Aloe barbadensis gel, and Allium sativum oil,23 and extracts of Miswak, Allium sativum, and Nigella sativa.14
However, contrary to the present research in which both the medicaments showed comparable clinical results, in a previous study Allium sativum oil exhibited significantly better clinical as well as radiographic results than that of Formocresol as non-vital pulpotomy medicament.1
In a histological evaluation, Allium sativum oil compared with Formocresol in permanent teeth,24 and with tri-antibiotic paste and Formocresol in primary teeth25 showed significantly better dentine bridge formation and healing potential. The results could not be compared to the current study as those were histological evaluations. However, the findings of these studies further support the potential use of Allium sativum oil as pulpotomy medicament.
The limitations of the study were the small sample size and short clinical follow-up period that may have effect on the study results. Furthermore, longer and variable sample transportation time might have influence on the viability of bacterial colonies.
CONCLUSION
In this study, the antibacterial effect of Allium sativum oil was evaluated and compared with Formocresol, against S. mutans and L. acidophilus, in terms of bacterial count (CFU/ml) in non-vital pulpotomy of primary molars. The results indicated comparable antibacterial effect. Additionally, the clinical outcomes of both medicaments were also comparable supporting the reliability of non-vital pulpotomy procedure itself. This concludes that Allium sativum oil can be used as an effective alternative to Formocresol in non-vital pulpotomy of primary molars.
DISCLOSURE:
This is an article derived from the thesis.
ETHICAL APPROVAL:
Ethical approval was obtained from Ethical Review Committee, Post-Graduate Medical Institute/ Ameer-ud-Din Medical College/ Lahore General Hospital, Lahore (AMC/ PGMI/ LGH/ Synopsis No./ 0081-19).
PATIENTS’ CONSENT:
Informed consent was obtained from patients’ parents.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
MM: Design investigations of work and write up.
SN: Conception, critical analysis, and data analysis and interpretation.
AZ, NB, AH: Drafting the manuscript.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Mohammad SG, Baroudi K. Assessment of the potential of allium sativum oil as a new medicament for non-vital pulpotomy of primary teeth. J Int Soc Prev Community Dent 2015; 5(4):314. doi: 10.4103/2231-0762.161762.
- Hargreaves, KM. Cohen's Pathways of the Pulp: South Asia Edition E-Book. 12th ed., Elsevier Health Sciences 2020; 992p.
- Nadelman P, Bedran N, Magno MB, Masterson D, de Castro AC, Maia LC. Premature loss of primary anterior teeth and its consequences to primary dental arch and speech pattern: A systematic review and meta‐analysis. Int J Paediatr Dent 2020; 30(6):687-712. doi:10.1111/ipd.12644
- Ritter V, Boushell W, Walter RS. Sturdevant's Art and Science of operative Dentistry. 7th ed., United States: Elsevier, St. Louis-Missouri 2018; 530.
- Ghazal TS, Levy SM, Childers NK, Carter KD, Caplan DJ, Warren JJ, et al. Mutans streptococci and dental caries: A new statistical modeling approach. Caries Res 2018; 52(3):246-52. doi: 10.1159/000486103.
- Mitrakul K, Vongsawan K, Watcharakirin W, Khererat P. Quantitative analysis of lactobacillus and enterococcus faecalis between irreversible pulpitis and pulp necrosis in primary teeth. Dent Res Oral Health 2019; 2(2):17-31. doi:10.26502/droh.008.
- Mohammad SG, Baroudi K. Bacteriological evaluation of Allium sativum oil as a new medicament for pulpotomy of primary teeth. J Int Soc Prev Community Dent 2015; 5(2): 125-30. doi: 10.4103/2231-0762.155738.
- Sebourn S, YU Q, Ritwik P. Pulpectomy versus extraction for the treatment of non-vital primary second molars: A retrospective chart review. J Clin Pediatr Dent 2020; 44(5):302-6. doi: 10.17796/1053-4625-44.5.3.
- Singh PH, Naorem H. Diagnosis and concepts of vital pulp therapy in primary teeth. EC Clin Med Case Rep 2020; 3:10.
- Surana P, Khandelwal A, Gopal R, Koppalkar RR, Aafreen S, Gupta S. Recent advances in pulpotomy medicament. Int J Med Oral Res 2021; 6:22-3. doi: 10.4103/ijmo.ijmo_11_20.
- Pannu R. Formocresol in dental domain: A Review. Int J Sci Res (IJSR) 2018; 8(9):719-22. https://www.ijsr.net/get abstract.php?paperid=ART20201100
- Kshirsagar MM, Dodamani AS, Karibasappa GN, Vishwakarma PK, Vathar JB Sonawane KR, et al. Anti-bacterial activity of garlic extract on cariogenic bacteria: An in vitro study. Ayu 2018; 39(3):165-8. doi: 10.4103/ ayu.AYU_193_16.
- Shooriabi M. Effects of allium sativum (Garlic) and its derivatives on oral diseases: A Narrative Review. J Res Dent Maxillofac Sci 2021; 6(1):36-44.
- Hashem SN, Fawzy MI, Mostafa MH. Comparative study of some natural materials versus traditional medicaments used for pulp treatment of primary teeth. Al-Azhar Dent J Girls 2019; 6(1):25-30. doi:10.21608/adjg.2019.5230. 1001.
- Roshan NM, Shigli AL, Deshpande SD. Microbiological evaluation of salivary Streptococcus mutans from children of age 5-7 years, pre- and post-atraumatic restorative treatment. Contemp Clin Dent 2010; 1(2):94-7. doi: 10.4103/0976-237X.68602.
- Karah N, Rafei R, Elamin W, Ghazy A, Abbara A, Hamze M, et al. Guideline for urine culture and biochemical identification of bacterial urinary pathogens in low-resource settings. Diagnostics (Basel) 2020; 10(10):832. doi:10.3390/diagnostics10100832.
- Bhujel N, Duggal M, Saini P, Day P. The effect of premature extraction of primary teeth on the subsequent need for orthodontic treatment. Eur Arch Paediatr Dent 2016; 17(6):423-34. doi: 10.1007/s40368-016-0247-7.
- Mahmood MA-K, Ali HE, Abdul-Kadher HK. The role of intracanal medicaments in inhibition of bacteria isolated from root canals of infected primary molars. Mustansiriya Dent J 2018; 14(1):92-8. doi:10.32828/mdj.v14i1.766.
- Imani Z, Imani Z, Basir L, Shayeste M, Abbasi Montazeri E, Rakhshan V. Antibacterial effects of chitosan, formocresol and CMCP as pulpectomy medicament on enterococcu-sfaecalis, staphylococcus aureus and streptococcu-smutans. Iran Endod J 2018; 13(3):342-50. doi: 10.22037/iej.v13i3.20791.
- Torbati M, Emamverdizadeh P, Torbati M, Maghalian M, Mirghafourvand M. Effect of garlic (Allium sativum) extract on salivary streptococcus mutans: A systematic review and meta-analysis. Pharm Sci 2021; 27(4):472-80. doi: 10.34172/PS.2021.37.
- Ghada Rezk Gomaa, Gehan G. Allam. Clinical and radiographic evaluation of the extract of allium sativum in pulpotomy of primary molars: A randomized clinical trial. Int J Dentistry Oral Sci 2020; 7(6):747-52. doi: 10.19070/ 2377-8075-20000147.
- Kahvand M, Mehran M, Haghgoo R, Faghihi T. Clinical and radiographic evaluation of Allium sativum oil (garlic oil) in comparison with formocresol in primary molar pulpotomy. J Int Soc Prev Community Dent 2019; 9(4):390-95. doi: 10.4103/jispcd.JISPCD_145_19.
- Abirami K, Ramkumar H, Senthil D. Clinical and radio-graphic evaluation of the efficacy of formocresol, allium sativum oil, and aloe barbadensis gel as pulpotomy medicaments in primary molars: A randomized controlled trial. Int J Clin Pediatr Dent 2020; 13(5):518-22. doi: 10.5005/jp-journals-10005-1802.
- Mohammad SG, Raheel SA, Baroudi K. Histological evaluation of allium sativum oil as a new medicament for pulp treatment of permanent teeth. J Contemp Dent Pract 2015; 16(2):85-90. doi: 10.5005/jp-journals-10024-1641.
- Mahfouz SM, Wahba OM. Comparative evaluation of pulpal response to tri-antibiotic paste and allium sativum with formacresol as pulpotomy medication in primary teeth: An in vivo study. Egypt Dent J 2019; 65(4):3131-42. doi:10. 21608/EDJ.2019.73990.