Comparative Effects of Azithromycin and Probiotics for Treatment of Acne Vulgaris
By Mushayada Irshad1, Akbar Waheed Syed1, Muhammad Waseem Shahid2, Uzma Naeem1, Mehreen Mirza1, Samarah Naeem3Affiliations
doi: 10.29271/jcpsp.2023.05.516ABSTRACT
Objectives: To measure the efficacy of oral probiotics when combined with a topical agent, and to compare the efficacy of Azithromycin, Probiotics, and their combination for the treatment of acne vulgaris.
Study Design: A quasi-experimental study.
Place and Duration of the Study: Pharmacology Department, IIMC in mutual collaboration with the Dermatology Department, Pak Emirates Military Hospital (PEMH), Rawalpindi, from September 2021 to August 2022.
Methodology: Seventy-five patients were enrolled in the study and were divided into 3 groups. Group A received Azithromycin (250mg oral on alternate days), Group B received probiotics i.e. Hi-Flora sachet (1 sachet oral daily), and Group C received both azithromycin (250mg oral on alternate days) and probiotics (Hi-Flora 1 sachet oral daily). The efficacy of three treatment regimens was measured by checking the difference in mean lesion count at the baseline and after 3 months of treatment and the percentage was calculated.
Results: All patients demonstrated significant improvement in total lesion count after treatment. In group A, the mean lesion count was reduced by 83.3%, in group B by 84.4%, and in group C, the reduction in mean lesion count was 90.3%.
Conclusion: Probiotics have equal efficacy to azithromycin, and their combination has shown synergistic effects for the treatment of acne vulgaris. Probiotics should be used in combination with azithromycin for the best results for the management of acne vulgaris.
Key Words: Acne vulgaris, Azithromycin, Probiotics, Efficacy.
INTRODUCTION
Acne is one of the most common skin diseases with greater impact on young population.1 It is generally presented as inflammatory or non-inflammatory lesions on the skin, which mainly involve face, but also chest and back.2 Worldwide, disease burden for acne vulgaris is 9.4% involving mainly pubertal acne while its prevalence is 5% in Pakistan.3 It is the third most prevalent skin condition in general population4 and more common in adult females (15-40 years of age) as compared to males.5
Acne is mainly classified on the basis of disease severity and lesion type. It is categorised into mild if there are <20 comedones, <15 inflammatory lesions or total lesion count is <30, moderate if there are 20–100 comedones, 15–50 inflammatory lesions or total lesion count ranges between 30–125, and severe if there are >5 pseudocysts, comedones >100, inflammatory lesions >50 or total lesion count exceeds 125.6
There are many treatments available for acne vulgaris. These could be local applications and oral agents; which include antibiotics, hormonal drugs, and isotretinoin.7 Commonly available topical agents are benzoyl peroxide (BPO), antibiotics, retinoids, or combination of any two. At times with these above-stated agents, many oral antibiotics are utilised for the treatment of acne such as tetracycline and macrolides.8
Azithromycin is the safest among macrolides with the efficacy similar to doxycycline and least interaction with other antimicrobials. So, azithromycin is the drug preferred these days as a better alternative to doxycycline by many dermatologists. Both of these protein synthesis inhibitors can treat acne with their bacteriostatic activity against Propionibacterium acnes with similar activity. Although at high dose, they have bactericidal action.9
“Probiotics are one of the emerging therapies for the treatment of acne vulgaris. The word probiotics is derived from the Latin word pro and the Greek word bios meaning “for life” and was first introduced by the German scientist Werner Kollath in 1953.10 They are defined by WHO as “live microorganisms which, when administered in adequate amounts, confer a health benefit on the host.” Probiotics should not be confused with pre-biotics which are basically carbohydrates or dietary fibers that help to feed and flourish the friendly bacteria already present in our gut.11
Probiotics are effective in the treatment of diarrhoea caused by any infection, use of antibiotics, and irritable bowel syndrome. They can boost the immune system and reduce inflammation and allergies. Poor gut health is related to unhealthy skin. Flushing waste from the body and having balanced gut microflora helps in maintaining fresh and clear skin, that’s why digestive issues can be the factor behind numerous skin-related issues.12 Many theories have been established with a fact that probiotics help improve the gut health and many inflammatory conditions. Scanty data are available on the use of probiotics as a separate modality for the treatment of acne vulgaris. Proposed mechanism of probiotics in acne is that they compete for attachment sites, feed on micro-nutrients that other pathogens also consume, modify the microbial flora by producing some antimicrobial compounds, and stimulate the immune response.13 Probiotics are commonly used for various other diseases e.g. viral and bacterial diarrhoea, irritable bowel syndrome, and other diseases involving the gut. They are also used as adjuvants to antibiotics to reduce their adverse effects. But their use in acne is quite uncommon.
The efficacy of azithromycin has been established already but it has not been compared with probiotics in acne patients. Also, the synergistic effect of both has not been determined in patients of acne vulgaris. The combination of azithromycin and probiotics has been used in the treatment of some other diseases like diarrhoea,14 impetigo15, and peri-implantitis16, but no documented details are available regarding the utilisation of this combination for the management of acne vulgaris.
The aim of this study was to evaluate and compare the effects of these drugs on acne patients.
METHODOLOGY
A quasi-experimental study was conducted in the outpatient Department of Dermatology, Pak Emirates Military Hospital, Rawalpindi, Pakistan in mutual collaboration with Pharmacology Department, IIMC, Riphah International University, Islamabad, Pakistan. The study was carried out after approval by the Institutional Review Committee (IRC) of the Islamic International Medical College (IIMC) with Ref. No. Riphah/IIMC/IRC/21/61 and Ethical Committee of the Pak Emirates Military Hospital (PEMH) with Ref. No. A/28/EC/337/2021. The study is registered with clinicaltrials.gov with the registration number NCT05629468. The data were collected from September 2021 till August 2022. Sample size was calculated to be 75 with Cochran formula with 5% prevalence of acne, z-score, and 5% margin of error. A convenient sampling technique was used. Patients with acne visiting the dermatology OPD of Pak Emirates Military Hospital who met the inclusion criteria were included by the study researchers. Written informed consent (both in English and Urdu) was taken from each participant. Male and female patients with mild to moderate acne aged between 15 to 35 years were included. However, patients with severe acne or any systemic inflammatory disease, patients taking any other oral treatment for acne, patients allergic to any drug used in the study, pregnant women, PCO patients, and patients taking any drug that can interact with azithromycin were excluded from this study.
The subjects were divided into 3 groups. Group A received tab. Azithromycin 250mg oral on alternate days for 3 months. Group B received Hi-Flora sachet orally every day for 3 months. Group C received tab. Azithromycin 250mg oral on alternate days and Hi-Flora sachet oral every day for 3 months. Those in group C were instructed to take probiotics for at least 2 hours after their azithromycin dose. All three groups received standard topical acne medication (benzoyl peroxide 4%) to be applied on lesions twice a day.
The outcome was measured by counting the total acne lesions. Lesions were counted at baseline (at the recruitment of the subject), and then patients were called for follow-up after 2, 4, 8, and 12 weeks, respectively. Decrease in the lesion count from baseline was observed and calculated at every follow-up. Data were analysed using SPSS.23 and required statistical tests were applied. Mean of lesion count at baseline and follow-ups were calculated. Percent decrease in the mean lesion count of groups A, B and C was calculated. Paired t-test was applied to compare mean differences before and after treatment within each group. One-way ANOVA was applied to observe group mean differences. A p-value of <0.05 was considered statistically significant. Categorical variables were expressed as counts and percentages and continuous variables were expressed as mean and SD.
RESULTS
In total of 75 patients, 25 (33.3%) were males and 50 (66.7%) were females with the mean age of 23+5 years. Out of the total patients, 26 (34.7%) patients were in the age bracket of 15-20 years, 22 (29.3%) patients in 21-25 years, 17 (22.7%) in 26-30 years, and 10 (13.3%) of the sample were in the age group of 31-35 years.
Mean of the total lesion count in groups A, B, and C at baseline, after 2, 4, 8 and 12 weeks are summarised in Figure 1.
The efficacy of treatment regimens in each group was measured by calculating the mean lesion count before and after treatment within the same group. Then mean differences and percent decrease in lesions were calculated. In group A, the mean lesion count was reduced by 83.3%, in group B by 84.4% and in group C by 90.3% as shown in the Table I.
To compare the mean values of total lesion count among these 3 groups one-way ANOVA was applied as shown in the Table II.
Few side effects were reported by patients taking probiotics i.e. mild nausea and bloating. Some patients taking azithromycin complained about mild diarrhoea that resolved on its own.
Table I: Paired t-test to compare mean values before and after treatment in each group.
|
|
Group A (Azithromycin) |
Group B (Probiotics) |
Group C (Azithromycin |
|
Mean lesion count Before treatment (Mean + SD) |
49.2 + 17 |
49.6 + 21 |
50.8 + 19 |
|
Mean lesion count After treatment (Mean + SD) |
8.2 + 3 |
7.7 + 4 |
4.9 + 1 |
|
Mean difference (Mean + SD) |
41.0 + 17 |
41.9 + 19 |
45.9 + 19 |
|
Percent decrease in |
83.3% |
84.4% |
90.3% |
|
p-value |
<0.001* |
<0.001* |
<0.001* |
Table II: ANOVA test showing the comparison of mean lesion count among all groups.
|
Groups |
Total lesion count after 12 weeks of treatment (Mean + SD) |
p-value |
|
Group A (Azithromycin) |
8.2 + 3 |
0.002* |
|
Group B (Probiotics) |
7.7 + 4 |
|
|
Group C (Azithromycin + probiotics) |
4.9 + 1 |
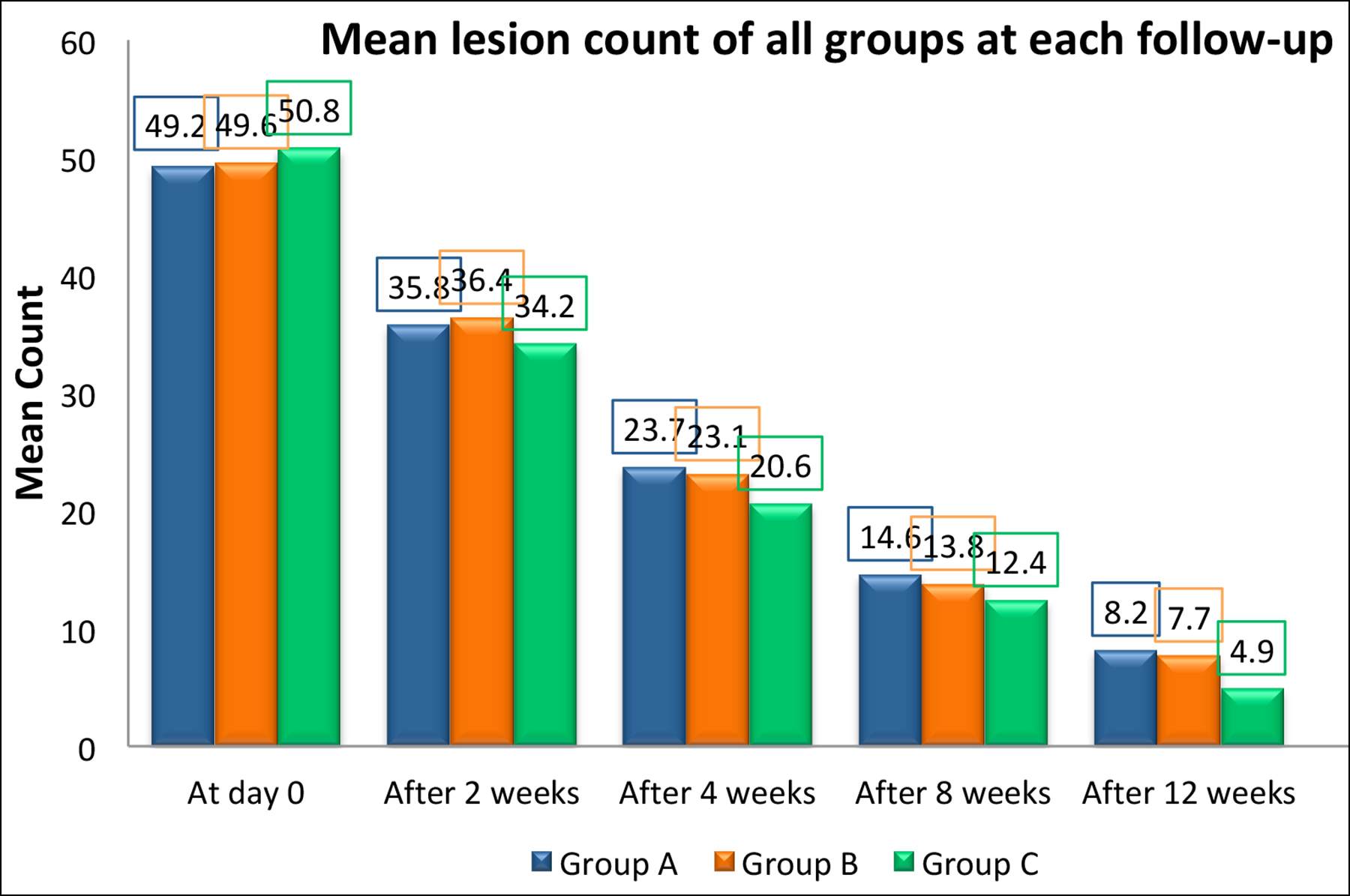 Figure 1: Bar chart showing mean lesion count in groups A, B and C at each visit.
Figure 1: Bar chart showing mean lesion count in groups A, B and C at each visit.
DISCUSSION
It has been established that probiotics which are known as good bacteria of gut directly inhibit the proliferation of acne-causing bacteria Propionibacterium acnes. In this study, the probiotics were given to the patients with acne as separate treatment modality, not as an adjuvant to other oral agents such as antibiotics.
In this research, out of total 75 patients, 25 (33.3%) were males and 50 (66.7%) were females. This predisposition of acne towards the female gender was also present in another study done by Arjel et al., where it was 62.5% females and 37.5% males.17 Another study done by Ahmed et al., also showed a more female proportion of the sample with 44% males and 56% females.18
The group A showed an overall good response with 83.3% decrease in mean lesion count after three months of treatment. These results are supported by Fernandez et al., who found similar efficacy of azithromycin in patients of acne vulgaris. They found that azithromycin reduced lesion count by 85% in their study group.19 The present results are supported by another study by Kapadia et al., who measured the efficacy of azithromycin in an open-label non-comparative study. It was a multicentred but single-armed study, and only 35 patients with moderate to severe nodulocystic acne were enrolled. The study showed remarkable improvement in 82.9% patients in the first 4 weeks.20
In group B, patients showed good efficacy with 84.4% decrease in total lesion count after 3 months of treatment. Although the literature on the use of probiotics for the treatment of acne vulgaris is quite scanty, few studies have been done. These results are in agreement with the pilot study conducted by Siver in 1961, cited by Bowe et al., to measure the efficacy of probiotics in acne patients, although it was a single-arm study with no control group.21 Jung et al., found a little bit lesser efficacy of probiotics than the current study although equal to antibiotic when they compared the efficacy of probiotics with minocycline in patients of mild to moderate acne, however, the synergistic effect of probiotics and minocycline was statistically significant, similar to the present study study.22
In group C, patients received combination of azithromycin + probiotics. The efficacy of this combination was found to be significantly higher than the other two groups as it reduced mean lesion count by 90.3%. This combination showed better efficacy than the other two regimens. The synergistic effect of this combination has been proven in some other diseases but not in acne; thus, making this study novel in approach. A study by Sudha et al., also showed synergistic effect of this combination in children with impetigo.15 This also proves that this combination is safe in children too because of its good tolerability and safety profile. This combination has been even used in children with obsessive compulsive disorder in a pilot study conducted by Murphy et al., and they also found out this combination to be better than probiotics alone.23 However, Hiroaki et al., found some different results when this combination was used in patients of peri-implantitis.16
Better tolerability and safety profile of these two drugs make them an effective and efficient choice for patients of acne vulgaris. Since, probiotics are proven to be equally efficacious to azithromycin in acne patients of this study, their use should be promoted to avoid side effects caused by excessive use of antibiotics. If the desired response is not achieved via monotherapy, combination of azithromycin and probiotics can be used because it has shown synergistic effect for the treatment of acne vulgaris.
It was a single-centred clinical trial involving only 75 patients. Separate effects on comedones, inflammatory lesions, and cysts/pseudocysts were not measured. Safety and tolerability of the drugs were not assessed by GASE scale. These parameters could not be measured due to cost and time limitations.
A multi-centred study can be conducted on a larger sample with randomisation and blinding. Probiotics can be compared with a different antibiotics in patients with acne. Different strains of probiotics can be used to measure their effect and efficacy on the treatment of acne. Generally, excessive and unnecessary use of antibiotics should be avoided in the management of acne and utilisation of probiotics should be encouraged.
CONCLUSION
Probiotics have shown very good efficacy when combined with a topical agent in the treatment of acne vulgaris. They have nearly equal efficacy to azithromycin in reducing the lesion count of acne patients. Azithromycin + probiotics have shown synergistic effect for the treatment of acne vulgaris, as combination is significantly more effective than the other two treatment regimens.
ETHICAL APPROVAL:
Ethical approval was obtained prior to the initiation of the research work from the Institutional Review Committee (IRC) of the Islamic International Medical College (IIMC) with Ref. No. Riphah/IIMC/IRC/21/61 and Ethical Committee of the Pak Emirates Military Hospital (PEMH) with Ref. No. A/28/EC/337/ 2021.
PATIENTS’ CONSENT:
Written informed consent (both in English and Urdu) were obtained from patients to participate in the study and to publish the data concerning this case.
COMPETING INTEREST:
The authors declare no competing interest.
AUTHORS’ CONTRIBUTION:
MI: Concept, design, acquisition, data collection, and data interpretation.
AWS: Concept, review, and approval.
MWS: Data collection and intellectual content.
UN: Data analysis, review, and approval.
MM: Review and intellectual content.
SN: Drafting and revision.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Bedout V De, Keri J, De Bedout V, Keri J. Acne therapy across time in the USA. Dermatol 2019; 81-8.
- Shalita AR. Acne: Clinical presentations. Clin Dermatol 2004; 22(5):385-6. doi: 10.1016/j.clindermatol.2004.03. 012.
- Babar O, Mobeen A. Prevalence and psychological impact of acne vulgaris in female undergraduate medical students of Rawalpindi and Islamabad, Pakistan. Cureus 2019; 11(9): e5772. doi: 10.7759/cureus.5722.
- Rea JN, Newhouse ML, Halil T. Skin disease in Lambeth. A community study of prevalence and use of medical care. Br J Prev Soc Med 1976; 30(2):107-14. doi: 10.1136/jech.30. 2.107.
- Skroza N, Tolino E, Mambrin A, Zuber S, Balduzzi V, Marchesiello A, et al. Adult acne versus adolescent acne: A retrospective study of 1,167 Patients. J Clin Aesthet Dermatol 2018; 11(1):21-5.
- Paul C. Rook’s Textbook of dermatology, 9th edn. Jane C. Sterling 2016; Chapter 90, Acne. 9th edition.
- Hauk L. Acne Vulgaris: Treatment guidelines from the AAD. Am Fam Physician 2017; 95(11):740-1.
- Mokhtari F, Faghihi G, Basiri A, Farhadi S, Nilforoushzadeh M, Behfar S. Comparison effect of azithromycin gel 2% with clindamycin gel 1% in patients with acne. Adv Biomed Res 2016; 5:72. doi: 10.4103/2277-9175.180641.
- Nischal K, Basavaraj H, Swaroop M, Agrawal D, Sathyanarayana B, Umashankar N. Nicolau syndrome: An iatrogenic cutaneous necrosis. J Cutan Aesthet Surg 2009; 2(2): 92-5. doi: 10.4103/0974-2077.58523.
- Gasbarrini G, Bonvicini F, Gramenzi A. Probiotics history. J Clin Gastroenterol 2016; 50 Suppl 2:S116-S9. doi: 10. 1097/MCG.0000000000000697.
- Sanders ME, Goh YJ, Klaenhammer TR. Probiotics and prebiotics. Food Microbiol Fundam Front 2019; 10(10): 831-54. doi: 10.1128/9781555819972.ch32.
- Chilicka K, Dzieńdziora-Urbińska I, Szyguła R, Asanova B, Nowicka D. Microbiome and probiotics in acne vulgaris-a narrative review. Life (Basel) 2022; 12(3):422. doi: 10. 3390/life12030422.
- Baldwin H, Tan J. Effects of diet on acne and its response to treatment. Am J Clin Dermatol 2021; 22(1):55-65. doi:10.1007/s40257-020-00542-y.
- Ahmadi M, Mohtasham N, Shamsizadeh A, Javaherizadeh H, Cheraghian B, Alizadeh M. Saccharomyces boulardii plus azithromycin is superior to azithromycin alone in reducing the duration of diarrhea in children with acute colitis: A double-blind randomised placebo-controlled trial. Immunopathol Persa 2022; 8(2):4-8. doi:10.34172/ipp. 2022.31397.
- Sudha G, Dharani, Nirmala P, Ramanathan R. and VS. Comparative study of efficacy and safety of azithromycin alone and in combination with probiotic in the treatment of impetigo in children. Int J Curr Pharm Res 2017; 9(6):52 doi:10.22159/ijcpr.2017v9i6.23429.
- Tada H, Masaki C, Tsuka S, Mukaibo T, Kondo Y, Hosokawa R. The effects of Lactobacillus reuteri probiotics combined with azithromycin on peri-implantitis: A randomized placebo-controlled study. J Prosthodont Res 2018; 62(1): 89-96 doi:10.1016/J.JPOR.2017.06.006.
- Arjel A, Pokhrel K, Sharma S. Efficacy of oral azithromycin versus doxycycline in the treatment of acne vulgaris. J Nepalgunj Med Coll 2021; 18(2):59-62 doi: 10.3126/ jngmc.v18i2.38908.
- Ahmad S, Aman S, Nadeem M, Hasnain Kazmi A. Efficacy and safety of oral azithromycin in the treatment of mild to moderate acne vulgaris. Ann 2011; 17(4):442–77.
- Fernandez-Obregon AC. Azithromycin for the treatment of acne. Int J Dermatol 2000; 39(1):45-50 doi:10.1046/j.1365- 4362.2000.00749.x.
- Kapadia N, Talib A. Acne treated successfully with azithromycin. Int J Dermatol 2004; 43(10):766-7. doi: 10. 1111/j.1365-4632.2004.02058.x.
- Bowe W, Patel NB, Logan AC. Acne vulgaris, probiotics and the gut-brain-skin axis: from anecdote to translational medicine. Benef Microbes 2014; 5(2):185-99. doi: 10.3920/BM2012.0060.
- Jung GW, Tse JE, Guiha I, Rao J. Prospective, randomized, open-label trial comparing the safety, efficacy, and tolerability of an acne treatment regimen with and without a probiotic supplement and minocycline in subjects with mild to moderate acne. J Cutan Med Surg 2013; 17(2): 114-22 doi:10.2310/7750.2012.12026.
- Murphy TK, Brennan EM, Johnco C, Parker-Athill EC, Miladinovic B, Storch EA, et al. A double-blind randomized placebo-controlled pilot study of azithromycin in youth with acute-onset obsessive-compulsive disorder. J Child Adolesc Psychopharmacol 2017; 27(7):640-51 doi:10.1089/cap. 2016.0190.