Colonic Metastasis from Primary Tonsillar Squamous Cell Carcinoma: A Rare Cause of Large Bowel Obstruction
By Tayfun Kaya1, Semra Demirli Atici1, Dudu Solakolu Kahraman2, Huseyin Salih Semiz3, Duygu Kahraman2Affiliations
doi: 10.29271/jcpsp.2022.08.S144ABSTRACT
Tonsillar squamous cell carcinoma is a subtype of head and neck cancer that rarely metastasizes to the colorectal system. Colonic metastasis secondary to primary tonsillar squamous cell carcinoma is a very rare clinical occurrence with unclear pathogenesis. Palliative chemo-radiation and surgery are recommended for this rare condition, which is generally seen in advanced stages.
Here, we aimed to report a case of a 70-year male who underwent palliative surgery due to the symptoms of mechanical bowel obstruction as a result of colonic metastasis of tonsillar carcinoma and review the relevant literature.
Key Words: Tonsillar carcinoma, Colon, Metastasis.
INTRODUCTION
Tonsillary cancer (TC), a subgroup of the head and neck tumour according to the pathological classification, is increasing in incidence, especially in the USA.1 TC spreads by the lymphatic and hematogenous pathways. TC mostly spreads to the cervical lymph nodes regionally. Distant spread of tumour is rarely seen in early-stage cancers, whereas advanced-stage cancers may metastasize to many organs beyond the cervical lymph nodes.1 Although squamous cell carcinomas (SCCs) of the head and neck region usually metastasize to the lungs, bones, and liver, they can rarely metastasize to colon.2
We, herein, present a case of a 70-year male who underwent palliative surgery due to the symptoms of mechanical bowel obstruction as a result of colonic metastasis of tonsillar carcinoma and review the relevant literature.
CASE REPORT
A 70-year male patient with a history of chronic ischemic heart disease and chronic obstructive pulmonary disease presented to the emergency service with complaints of abdominal pain, nausea, and vomiting for two days. He had a history of smoking one pack of cigarettes per day for 50 years and alcohol.
He had no history of abdominal surgery. He had diagnosed with the left palatine TC by the otolaryngology team with the help of a biopsy. He had cT3N3bM0 stage TC supported by positron emission tomography (PET) study five months ago. He refused any medical treatment such as chemotherapy and radiotherapy. Serology tests of the patients were negative for Epstein-Barr Virus (EBV). On physical examination, he had a significant tenderness, rebound tenderness in all quadrants of the abdomen, and abdominal distension. His abdominal X-ray was compatible with mechanical bowel obstruction. He had a significant leucocytosis with Total Leucocyte Count (TLC) of 24,500/mm3 (normal range: 4,200-10,600/mm3) and high lactate at 7.4 mmol/L (normal range: 0.3-1.3 mmol/L). The patient underwent an urgent explorative laparotomy with a presumptive diagnosis of mechanical bowel obstruction. A cecal mass as a cause of obstructive bowel obstruction was detected during laparotomy and due to the perioperative obstructive findings, we performed a right hemicolectomy with a Mikulicz ileostomy. The patient was discharged on the 7th postoperative day without any complications.
The histopathological examination of right hemicolectomy material was compatible with metastasis of tonsillar non-keratinized SCC. In the macroscopic examination of the resection material, a nodular, dirty white-colored, 5×3×2.5 cm mass was observed involving submucosal and serosal layers of the cecum (Figure 1). The tumor had signs of invasion into the submucosa, muscularis propria and serosa. Signs of lymphovascular and perineural invasion were present in the samples. The tumour showed a histological pattern of solid islands with necrosis in the center. More than 20 mitotic figures were counted in 10 high-magnification areas. Poorly differentiated SCC with metastasis to the cecum consists of pleomorphic cells in sheets, as in this case (Figure 2a, Figure 2b). There were tumor metastases in 2 out of 15 lymph nodes sampled from pericolic adipose tissue. Immunohistochemical examination pointed out that tumor cells were positive for p40, p63, EMA, and high molecular-weight cytokeratin (HMWCK) (Figure 2c). Additional immunohistochemical staining analyzes were performed to exclude other possible origins of SCC. Tumor cells were negative for CK7, CK20, CDX2, synaptophysin, and chromogranin.
 Figure 1: Gross appearance of metastatic carcinoma.
Figure 1: Gross appearance of metastatic carcinoma.
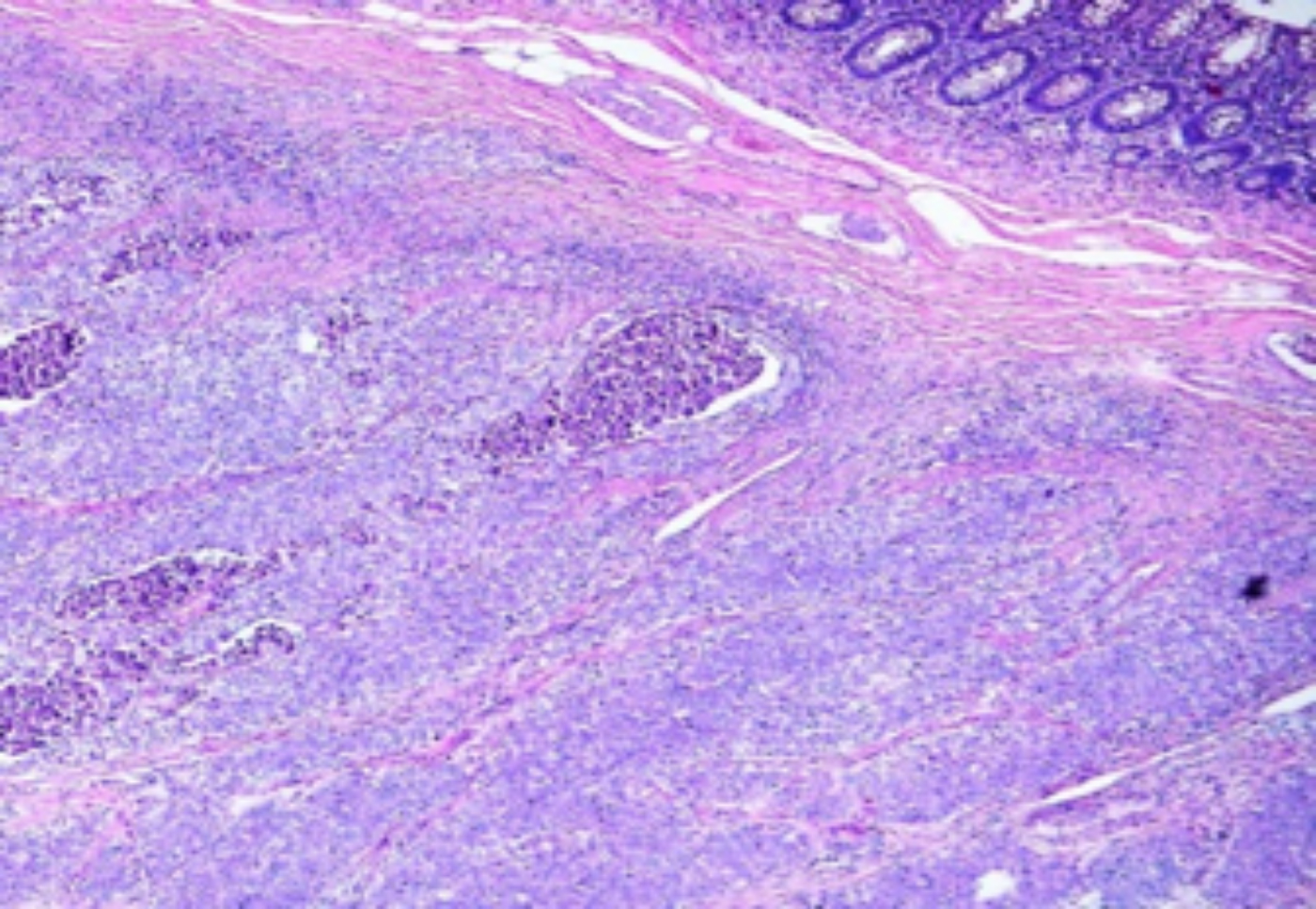 Figure 2a: Histopathology images of tonsillar carcinoma colon (cecum) metastasis with associated normal colon tissue (H&E, ×40).
Figure 2a: Histopathology images of tonsillar carcinoma colon (cecum) metastasis with associated normal colon tissue (H&E, ×40).
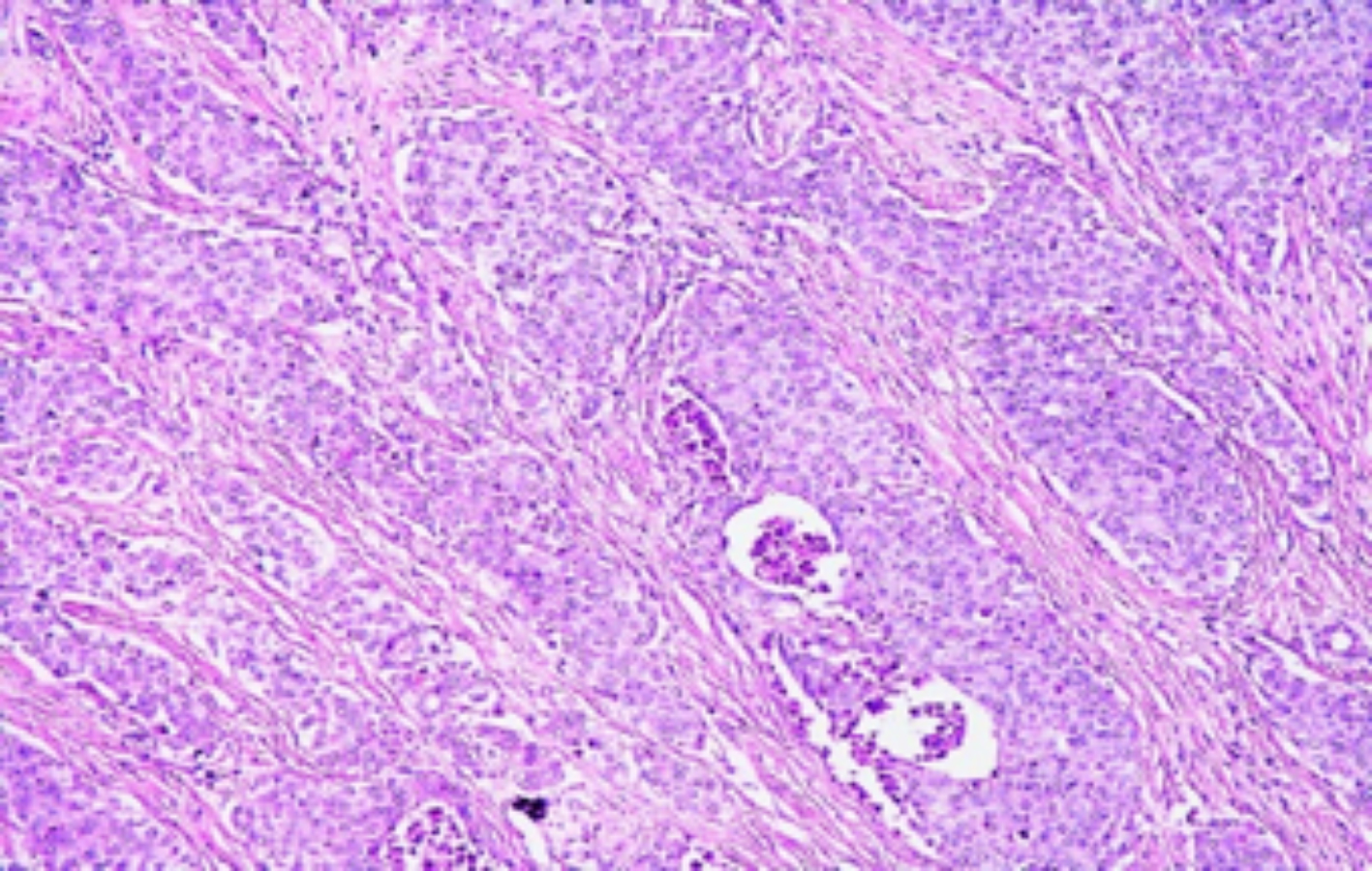 Figure 2b: Poorly differentiated squamous cell carcinoma metastatic to cecum consists of pleomorphic cells in sheets. No keratin pearls or glandular pattern is seen (H&E, ×200).
Figure 2b: Poorly differentiated squamous cell carcinoma metastatic to cecum consists of pleomorphic cells in sheets. No keratin pearls or glandular pattern is seen (H&E, ×200).
DISCUSSION
TC is a subtype of head and neck tumor and its incidence has increased in recent years.1,2 Although the incidence of the disease varies depending on the region and etiology, increased human papilloma virus (HPV) positive cases and smoking have been reported to contribute to increased incidence of TC.1
Local invasion and neck node involvement in head and neck tumors is the usual form of spread.1,2 In addition, lung, liver, and bone are usual areas of distant metastases in these tumors.2 TC rarely involves the colorectal system and until now, one case of colon metastasis due to TC has been reported in the literature.2
Metastasis of primary extra-abdominal cancers to the colorectal system is seen infrequently. Carcinomas that metastasize from the primary tumors in the extra-abdominal region to the colon include malignant melanoma, lobular breast carcinoma, and lung cancer .2 Small bowel metastasis due to TC is even rarer, and has been attributed to hematogenous dissemination or an abnormality of the lympho-vascular drainage.3
Physical examination and laboratory testing are not specific for diagnosing bowel metastasis.2,3 Due to nonspecific findings associated with gastrointestinal metastases, these are often detected when they are at an advanced stage or during autopsy.2,3 In the literature, large bowel metastasis caused by primary malignancy belonging to many organs such as the small intestine, ovaries, stomach, and pancreas, caused by transperitoneal seeding is observed.3 However, large bowel metastasis from TC has not been reported, except for one case which was diagnosed by colonoscopic examination.2 Tariq et al. reported the above case for the first time in the literature in a 57-year male patient.2
Poorly differentiated SCC in the colon is distinguished from primary adenocarcinomas by clinical and immunohistochemical findings.4 In our case, the histomorphological features of the tumor in the cecum were similar to the tumor in the first biopsy material taken from the tonsil. Immunohistochemically, both tumors were HMWCK, p63, and CK5/6 positive. Since the tumor in the colon was immunohistochemically negative with CK20, CDX2, the primary poorly differentiated adenocarcinoma of the colon was not considered a diagnosis. The incidence of colonic SCCs was reported to be 0.1-0.25 per 1,000 colorectal cancers.4,5 These tumors are metastatic or primary.4,5 Metastatic and primary SCCs are immunohistochemically positive for p63 and CK 5/6.5 The tumor detected in the colon was p63 and CK5/6-positive in our case. It was interpreted as metastasis because our patient had previously been diagnosed poorly differentiated tonsillar SCC, and histomorphologically, no evidence of squamous metaplasia or epithelial-related squamous tumor development in the colonic mucosa was seen.
Intestinal metastasis is frequently seen in advanced-stage patients due to SCC of tonsillar origin. Patients may be completely asymptomatic or manifest with atypical gastrointestinal symptoms such as nonspecific abdominal pain and bloating.2,3 Patients may also show symptoms such as tachycardia and weakness secondary to developing anemia and hematochezia, which may occur secondary to bleeding from the metastatic colonic mass. In addition, colonic metastasis secondary to TC may be diagnosed for the first time with complications such as obstruction, perforation, and bleeding secondary to colonic mass.3
As there is no definitive treatment for metastatic colorectal cancers due to head and neck carcinomas, these patients are generally not suitable for surgical treatment methods.2 In isolated metastases secondary to colorectal cancers, local surgical resection may benefit some patients, but the prognosis is generally poor in metastases of primary non-colorectal cancers.6 In such advanced disease states, the primary goal is to improve the quality of life for these patients by planning to reduce their symptoms. Currently, there is no standard therapeutic regimen. However, in most patients, palliative chemo-radiation therapy and in very rare cases, surgical resection may be used.2,6 Chemotherapy and palliative radiation therapy have all been proposed as treatment modalities for metastatic tumors, with surgery only indicated in rare situations such in our case due to symptoms of obstruction, perforation or bleeding.3,6
In conclusion, obstruction, perforation, and bleeding may be the presenting sign of possible metastatic intestinal disease secondary to TC. Evaluating such metastatic patients with early colonoscopy may reduce complications that may occur. Larger case series are needed to better understand the pathophysiology of this rare disease, facilitating an early diagnosis and treatment.
PATIENT’S CONSENT:
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
TK, SDA, DSK, HSS: Reviewed the literature.
DSK: Performed the histopathological examination and prepared the histopathological figures.
SDA: Designed the study, participated the surgical treatment of the patient, and prepared the manuscript.
TK: Arranged the figures.
SDA, DK: Edited and reviewed the manuscript.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Coca-Pelaz A, Rodrigo JP, Suarez C. Clinicopathologic analysis and predictive factors for distant metastases in patients with head and neck squamous cell carcinomas. Head Neck 2012; 34(6):771-75. doi: 10.1002/hed.21804.
- Tariq H, Kamal MU, Mehershahi S, Saad M, Azam S, Kumar K, et al. A rare case of colonic metastases from tonsillar carcinoma: Case report and review of literature. World J Oncol 2018; 9(1):35-37. doi: 10.14740/wjon1073w.
- Dwivedi RC, Kazi R, Agrawal N, Chisholm E, St Rose S, Elmiyeh B, et al. Comprehensive review of small bowel metastasis from head and neck squamous cell carcinoma. Oral Oncol 2010; 46(5):330-5. doi.10.1016/j.oral ocncology.2010.01.013.
- Shannon Greenberg, Jared C. Inman, Esther Yung, and Evelyn B. Choo. Colon mass: Arare site of metastasis for squamous cell cancer of the head and neck. J Surgical Case Reports 2020; 2020(2):391. doi: 10.1093/jscr/rjz391.
- Abdelqader A, Jabaji R, Albugeaey M, Palese C. Squamous cell carcinoma of the ascending colon: Two cases. J Community Hosp 2017; 7(1):53-5. doi: 10.1080/2000 9666.2017.1309339.
- Hauswald H, Simon C, Hecht S, Debus J, Lindel K. Longterm outcome and patterns of failure in patients with advanced head and neck cancer. Radiat Oncol 2011; 6:70 doi: 10.1186/1748-717X-6-70.