Co-existence of Papillary and Medullary Thyroid Carcinoma: Reports of Three Cases
By Murat Calapkulu1, Ozen Oz Gul2, Soner Cander2, Muhammed Fatih Sagiroglu3, Ozlem Saraydaroglu4, Erdinc Erturk2, Canan Ersoy2Affiliations
doi: 10.29271/jcpsp.2022.08.S156ABSTRACT
Medullary thyroid carcinoma (MTC) and papillary thyroid carcinoma (PTC) are two different types of thyroid carcinoma. They have different features in terms of cellular origin, histopathology, clinical features, prevalence, and prognosis. PTC originates from follicular cells, while MTC from parafollicular cells. MTC and PTC co-existence is a rare phenomenon and occurs in less than 1% of all thyroid tumors. We report three cases with coexistent MTC and PTC in the same thyroid. The papillary component was dominant in two cases and the medullary in one case. While the first case was given radioactive iodine therapy, the third was treated with vandetanib. The second case was followed up postoperatively and did not receive treatment other than levothyroxine replacement. The co-existence of these tumors requires a different clinical approach in treatment and follow-up, depending on which type is dominant.
Key Words: Mixed thyroid carcinoma, Papillary thyroid carcinoma, Medullary thyroid carcinoma.
INTRODUCTION
Medullary thyroid carcinoma (MTC) and papillary thyroid carcinoma (PTC) are two different types of thyroid carcinomas, having different origins and histopathological appearances. PTC constitutes the vast majority of all thyroid cancers (85%).1 It originates from the follicular cells of the endoderm origin and produces thyroglobulin.1 MTC accounts for 1-2% of cases of thyroid cancer and originates from parafollicular C cells.2 MTC synthesizes large amount of calcitonin, its biological marker.2 Although 50% of PTC cases spread to the neck lymph nodes, lymphatic spread does not affect survival, unlike other thyroid cancers.1 Distant metastases can develop in up to 15% of PTC cases and are most common in the lung, bone, and liver.1 The mortality rate in PTC is 2% at a 5-year follow-up.1 MTC has two types, sporadic and hereditary or familial type.2
Multiple endocrine neoplasia (MEN) 2A is a hereditary syndrome that is transmitted in an autosomal dominant pattern, which comprises of familial MTC, pheochromocytoma and parathyroid hyperplasia.2 The prognosis of MTC is worse compared to PTC.3
It is a rare phenomenon to see MTC and PTC simultaneously which occurs in less than 1% of all thyroid tumors.4 Biscolla et al. reported that 13.8% of MTC patients had simultaneous PTC.3 Kim et al. reported that 19% of MTC patients had simultaneous PTC.5 Progression and prognosis are similar to the predominant component of the tumor.6 Radioactive iodine (RAI) therapy can be used for the follicular components and metastases of the primary tumor. Unfortunately, although systemic RAI therapy is applied for the papillary component of these tumors, this treatment is not effective in the medullary component. The current report aimed to review the literature by presenting 3 cases with coexistent MTC and PTC.
CASE REPORT
Case 1: A 63-year woman was admitted to our centre for thyroid nodules. An enlarged thyroid gland with the two nodules in the right lobe (10.3 mm and 14.4 mm in the greatest dimensions) was detected in thyroid ultrasound (US). Thyroid function tests, serum calcium, parathormone, and carcinoembryonic antigen (CEA) levels were in the normal range (NR). Basal serum calcitonin was above the normal range as 139 pg/ml (NR: <11.5 pg/ml). Fine-needle aspiration biopsy (FNAB) was performed on the large nodule, and the result was suspicious for malignancy (Table I). A total thyroidectomy was performed. The histopathological evaluation revealed medullary microcarcinoma (6 mm) and a tall cell variant of PTC (15 mm) in the right thyroid lobe (Figure 1). An abdominal imaging scan found no adrenal nodules and urinary catecholamine; metanephrine levels were normal. The postoperative thyroid-stimulating hormone (TSH) was 40 mIU/ml (NR: 0.3-4.9 mIU/ml), while calcitonin was <2 pg/ml and thyroglobulin, 4.06 µg/L. RAI therapy was given as an adjuvant treatment after thyroidectomy.
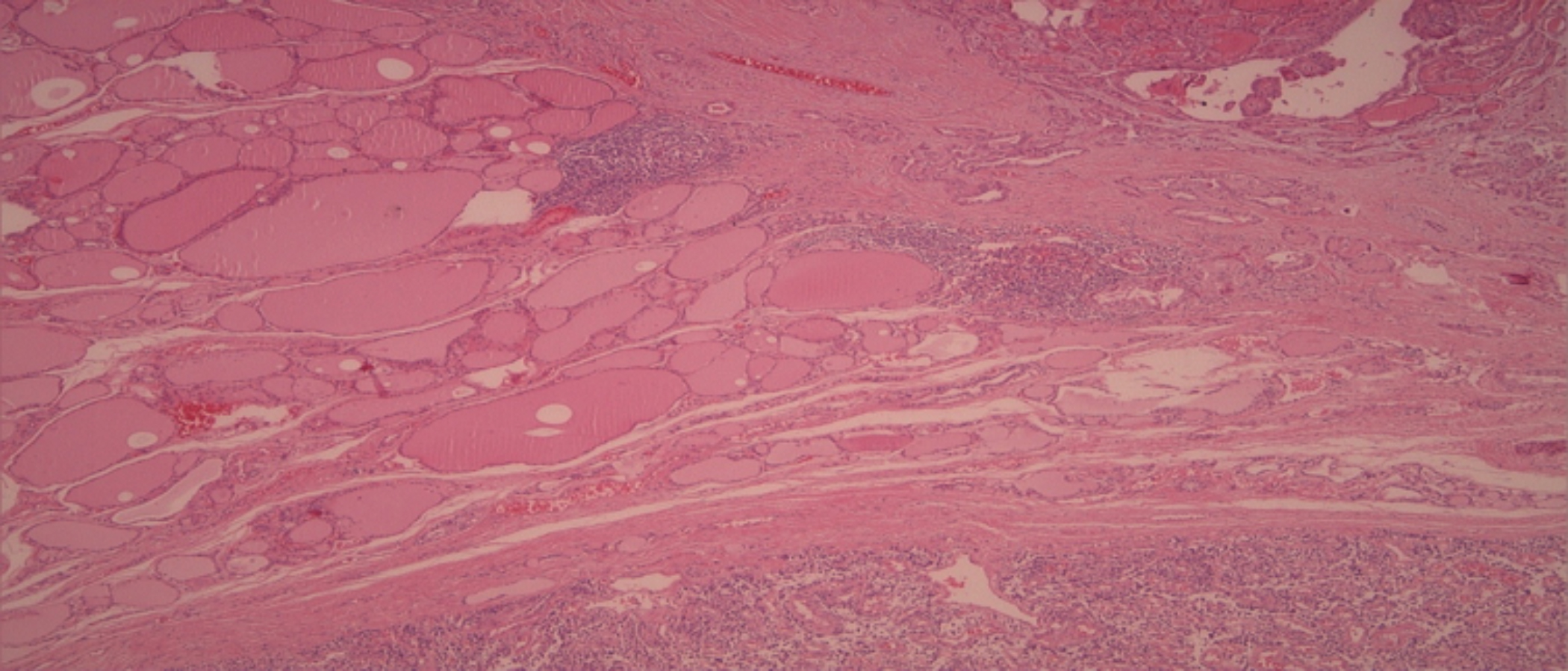 Figure 1: Histopathology of Case 1. Papillary thyroid carcinoma (PTC) and medullary thyroid carcinoma (MTC) are clearly separated by normal thyroid tissue (HE, ×100).
Figure 1: Histopathology of Case 1. Papillary thyroid carcinoma (PTC) and medullary thyroid carcinoma (MTC) are clearly separated by normal thyroid tissue (HE, ×100).
Case 2: An 81-year woman on follow-up for nodular goiter, was admitted to our center with the complaint of weight loss and palpitations. An enlarged thyroid gland with a nodule in the right lobe (38 mm in greatest dimension) was detected in the US. The TSH level was lower than the NR (NR: <0.0025 mIU/ml). The scintigraphic features revealed increased uptake in the right lobe and decreased uptake in the left lobe. FNAB was performed due to the large size of the nodule, and the result was suspicious for malignancy. The patient underwent right lobectomy. The histopathological evaluation of the excised material revealed medullary microcarcinoma (tumor diameter 3 mm). The complementary thyroidectomy was performed. Pathological evaluation revealed a 4 mm papillary microcarcinoma in the left lobe (Table I). Extrathyroidal extension and vascular invasion were not detected. RAI or systemic therapy was not given due to the low-risk PTC and MTC.
Case 3: A 60-year man was admitted to our center with the complaint of neck swelling. An enlarged thyroid gland with a microcalcified nodule (greatest dimension 25 mm) was detected in the right lobe in the US. The serum calcitonin was 11383 pg/ml, while TSH was within normal limits. FNAB disclosed a proliferative lesion suspicious for MTC. The patient underwent a total thyroidectomy, level VI-VII, and central neck dissection. The histopathological evaluation was reported as MTC (tumor diameter 30 mm) in the right lobe and papillary microcarcinoma (tumor diameter 4 mm) in the left lobe (Table I). Furthermore, pathological evaluation of lymph nodes revealed 10 lymph node-positive for metastases of the MTC. The postoperative calcitonin was 1892 pg/ml. Urine catecholamine levels were normal, and RET mutation was negative. Postoperative abdominal Magnetic Resonance Imaging (MRI) showed multiple metastatic lesions in the liver. The patient was given vandetanib treatment.
DISCUSSION
The co-existence of PTC and MTC is a rare condition and can be observed in two settings, a mixed tumor showing dual differentiation, or a collision tumor with well-defined components at distinct locations. This association is generally presented as case reports in the literature. MTC constitutes approximately 1-2% of all thyroid carcinomas, and 3-5% of these cases have a follicular or papillary cancer component.2,6 MTC arises from the parafollicular C cells. MTC has a great ability to exhibit unusual histological features such as follicular, papillary, trabecular, oxyphilic, anaplastic, and squamous differentiation. Although the ability of MTC to mimic other tumors sometimes cause confusion when making a morphological diagnosis, immunohistochemical studies usually confirm the parafollicular origin of the tumor. While MTC is negative for immunohistochemical stained thyroglobulin, but calcitonin and CEA are commonly positive. On the contrary, PTC stains positive for thyroglobulin.7
Collisional PTC and MTC are rare phenomenons with an incidence of <0.5% of all thyroid tumors. Various hypotheses have been proposed to clarify the histogenesis of collisional PTC and MTC. Most researchers believe that the association of MTC and PTC occurs as a result of the transformation of stem cells into neoplastic cells.8 These hypotheses are supported by studies showing co-expression of calcitonin and thyroglobulin in tumor cells.8 An alternative hypothesis is the presence of a common oncogenic stimulus that leads to the simultaneous transformation of both C cell and follicular progenitor stem cells and the development of follicular and medullary components.9 Volante et al. reported that the co-existence of medullary and follicular carcinoma is derived from the 2 different cells through detailed molecular analysis. They have demonstrated a new hypothesis, called the ̎ hostage hypothesis ̏ 9 Primarily, the neoplastic have transformation of parafollicular C cells will lead to the development of an MTC with entrapment of normal follicles around it. The microenvironment provided by the MTC cells would subsequently stimulate the proliferation of trapped follicular cells and ultimately lead to hyperplastic and adenomatous follicles, which will lead to neoplastic tissue.9 However, these hypotheses have not successfully answered all of the controversial questions about the formation of this co-existence.
In many cases, lymph node metastases have been reported at the time of diagnosis. Lymph node metastases can be composed of a single tumor or may have different morphological features such as the mixture of the both components in the same lymph node.5,6 Distant metastases are commonly seen in the lung, mediastinum, bone, and liver.6 In the present series, distant metastases were not detected in the first and second cases, while in the third case, metastasis was detected in the cervical lymph nodes and liver.
Table I: The clinical and histopathological characteristics of the three cases with co-existence of medullary and papillary thyroid carcinomas.|
Case No. |
Age (years) |
Gender |
FNAB |
Size |
RAI T. |
Chemo |
|
Case 1 |
63 |
Female |
SM |
6 mm MTC, 15 mm PTC |
pos |
neg |
|
Case 2 |
81 |
Female |
SM |
3 mm MTC, 4mm PTC |
neg |
neg |
|
Case 3 |
60 |
Male |
SM |
30 mm MTC, 4mm PTC |
neg |
pos |
|
FNAB: Fine-needle aspiration biopsy; RAI T: Radioactive iodine therapy; Chemo: Chemotherapy; SM: Suspicious for malignancy; MTC: Medullary thyroid carcinoma; PTC: Papillary thyroid carcinoma; pos: Positive; neg: Negative. |
||||||
Early diagnosis is important in co-existent of MTC and PTC. This association generally occurs in middle-aged people, and the most common symptom is neck swelling. It is necessary to support cytological diagnosis with serum calcitonin and thyroglobulin levels, and immunohistochemical examinations are required for an accurate diagnosis. Some cases with follicular components may be given RAI therapy. Machens et al. reported that the rate of MTC in separate foci among the patients with PTC was 2.6%.10 Biscolla et al. reported simultaneous PTC in 27 of 196 (13.8%) MTC cases.7 In the present study, in the first case, the PTC diameter was 15 mm, and the MTC diameter was 6 mm. Therefore, RAI treatment was given after surgery. In the second case, post-operative follow-up was planned because the tumor sizes were <5 mm and had low-risk factors. In our third case, systemic vandetanib was given because distant metastases were detected. Since the papillary component was less than 5 mm, RAI treatment was not given.
In conclusion, MTC-PTC co-existence is a rare clinical condition, and a definitive diagnosis of this rare association is essential for adequate treatment of the patient. Accurate diagnosis is necessary due to its prognostic implications and treatment decisions. Endocrinologists, surgeons, and pathologists should keep in mind the possible co-existence of these tumors.
PATIENTS’ CONSENT:
Informed consent was obtained from the patients to publish this case report.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
MC: Wrote the manuscript.
MC, OOG, SC, MFS: Participated in data collection.
OS: Participated in figure collection.
MC, OOG, CE: Contributed to the Discussion.
MC, OOG, CE, SC, EE: Contributed to study design, reviewed and edited the manuscript.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016; 26(1): 1-33. doi: 10.1089/thy.2015.0020.
- Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American thyroid association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015; 25(6):567. doi: 10.1089/thy.2014.0335.
- Biscolla RP, Ugolini C, Sculli M, Bottici V, Grazia Castagna M, Romei C, et al. Medullary and papillary tumors are frequently associated in the same thyroid gland without evidence of reciprocal influence in their biologic behavior. Thyroid Off J Am Thyroid Assoc 2004; 14(11):946-52. doi: 10.1089/thy.2004.14.946.
- Sizemore GW 1987 medullary carcinoma of the thyroid gland. Semin Oncol 1987; 14(3):306-14. PMID: 2888195.
- Kim WG, Gong G, Kim EY, Kim TY, Hong SJ, Kim WB, et al. Concurrent occurrence of medullary thyroid carcinoma and papillary thyroid carcinoma in the same thyroid should be considered as coincidental. Clin Endocrinol (Oxf) 2010; 72(2):256-63. doi: 10.1111/j.1365-2265.2009.03622.x.
- Papotti M, Negro F, Carney JA, Bussolati G, Lloyd RV. Mixed medullary-follicular carcinoma of the thyroid. A morphological, immunohistochemical and in situ hybridisation analysis of 11 cases. Virchows Arch Int J Pathol 1997; 430(5): 397-405. doi: 10.1007/s00428005 0049.
- Nangue C, Bron L, Portmann L, et al. Mixed medullary-papillary carcinoma of the thyroid: Report of a case and review of the literature. 2009. (doi: 10.1002/hed.20984)
- Noel M, Delehaye MC, Segond N, Lasmoles F, Caillou B, Gardet P, et al. Study of calcitonin and thyroglobulin gene expression in human mixed follicular and medullary thyroid carcinoma. Thyroid 1991; 1(3):249-56. doi: 0.1089/thy. 1991.1.249.
- Volante M, Papotti M, Roth J, Saremaslani P, Speel EJ, Lloyd RV, et al. Mixed medullary-follicular thyroid carcinoma: Molecular evidence for a dual origin of tumor components. Am J Pathol 1999; 155(5):1499-509. doi: 10.1016/S0002- 9440(10)65465-X.
- Machens A, Ukkat J, Hauptmann S, Dralle H. Abnormal carcinoembryonic antigen levels and medullary thyroid cancer progression: A multivariate analysis. Arch Surg 2007; 142(3):289-93; discussion 294. doi: 10.1001/ archsurg. 142.3.289.