Cardiac Troponin-I, A Biomarker for Predicting COVID-Induced Myocardial Damage Prognosis
By Abdul Rehman1, Shagufta Yousuf1, Ghulam Rasool Maken2, Syed Rehan Asghar Naqvi1, Ghulam Murtaza3, Abeera Ahmad4Affiliations
doi: 10.29271/jcpsp.2023.05.498ABSTRACT
Objective: To analyse the utility of cardiac Troponin-I as a prognostic marker in COVID-19-induced myocardial injury.
Study Design: A descriptive study.
Place and Duration of the Study: COVID Intensive Therapeutic Unit (ITC) and Pathology Department, Combined Military Hospital (CMH), Malir, Karachi, from September 2021 to February 2022.
Methodology: Patients with chest pain, who tested positive for COVID-19 by real-time PCR, were inducted. Blood samples were screened for inflammatory and cardiac biomarkers. The levels of cardiac Troponin I (cTn-I) were categorised as normal (99th percentile = ≤0.01 ng/ml), raised (5 times the 99th percentile = >0.01 ng/ml), and markedly raised (>10 times the 99th percentile = >10 ng/ml) based on serial monitoring over a duration of 6-8 hours.
Results: Out of a total of 104 patients, the mean age was 48 ± 15.94 years; 78 (75%) were males and 26 (25%) were females. The mean levels of cardiac Troponin I (cTn-I) were 1.91 ng/ml, C-reactive protein (CRP) was 85 mg/l, Interleukin-6 (IL-6) was 43.3 ng/ml, Procalcitonin (PCT) was 1.40 ng/ml, Creatinine Kinase (CK) was 203 U/l, CK MB was 31 U/l, and Ferritin was 471 ng/ml. Forty-four (42.4%) had normal cTn-I levels, 38 (36.5%) had raised levels, and 22 (21.1%) had markedly raised levels. A persistent rising pattern of cTn-I with a maximum rise up to 30 ng/ml was observed in 16 patients (15.3%) labelled as myocarditis, while only 8 (7.6%) showed a rise-fall pattern. Cardiac Tn-I and CRP were significantly higher in patients with myocarditis (p <0.01). Six out of 104 patients (5.7%) died due to COVID- induced myocardial injury all having raised cTn-I.
Conclusion: Cardiac Troponin-I is an effective biomarker for measuring myocardial injury in COVID-19 patients and can be an independent predictor to assess for severity of cardiac injury than other inflammatory markers in COVID-19.
Key Words: COVID-19, Cardiac Troponin I, Inflammatory markers, Myocardial injury, Prognosis.
INTRODUCTION
Cardiovascular involvement as a complication of SARS-CoV-2, the virus responsible for COVID-19, has been a topic of extensive research. Several proposed factors may contribute to this complication, including direct injury to the heart through ACE-2 receptors expressed in the heart.1 Other factors include the surge of cytokines, acute respiratory distress syndrome (ARDS) induced hypoxia, stress cardiomyopathy, ischemic injury due to cardiac microvascular dysfunction, vasculitis, and other ischemic cardiac manifestations.2
These factors may result in myocardial injury, as evidenced by elevated levels of cardiac Troponin-I (cTn-I), a myocardial regulatory protein with high specificity and considered the gold standard for early diagnosis of COVID-19-induced myocardial injury.3
Pre-existing cardiovascular health is a predictive factor for the risk of severe disease in COVID-19 patients. Serum cTn-I has been identified as an independent predictive biomarker of induced cardiovascular disease (CVD) severity and mortality.4 Elevated cTn-I levels above the 99th percentile have been associated with a low survival rate, highlighting the association of CVD severity in the presence of COVID-19.5 In addition to cTn-I, other inflammatory and cardiac biomarkers such as C-reactive protein (CRP), Interleukin 6 (IL-6), Ferritin, Procalcitonin (PCT), creatinine Kinase (CK), CKMB, N terminal pro-brain natriuretic peptide (NT-Pro-BNP), and lactate dehydrogenase (LDH) were elevated in COVID-19 positive patients upon admission.6 Rising levels of urea and creatinine have also been associated with adverse outcomes in COVID-19-induced myocardial injury.6 Notably, COVID-19-induced myocardial injury may also occur in pediatric patients, albeit rare. Cases of adolescents presenting with complete heart block myocarditis and elevated Troponin I level, along with severely depressed cardiac function, have been reported.7 Additionally, other viral illnesses caused by coronaviruses, such as Middle Eastern Respiratory Syndrome (MERS), have shown cardiac involvement with arrhythmic episodes, acute heart failure, and cardiac arrest.8 It is believed that adrenergic hyperactivation in response to respiratory difficulty and psychological stress associated with COVID-19 may result in ventricular dysfunction characteristic of Tako-Tsubo syndrome or acute myocarditis, which may present as Tako-Tsubo syndrome in some cases.9 Furthermore, SARS-CoV-2 may cause direct damage to the heart, including myocarditis with significant impairment of cardiac contractility, as well as affect the pericardium with the formation of an effusion that may impair cardiac function.10 Recently, SARS-CoV-2 was discovered on a myocardial biopsy in a patient with severe myocarditis followed by cardiac shock.11 Specifically, the aim was to identify the optimal diagnostic and monitoring strategies for cardiovascular involvement in COVID-19 patients using cTn-I levels and develop targeted interventions to prevent and manage COVID-19-related cardiovascular complications based on cTn-I as a prognostic marker. By addressing this objective, one can enhance the understanding of the prognostic value of cTn-I in COVID-19-induced myocardial injury and improve patient outcomes. The objective of this research was to investigate the role of cTn-I as a predictor of COVID-19-induced myocardial injury and prognosis.
METHODOLOGY
This study was conducted at the Combined Military Hospital (CMH) located in Malir Cantonment, Karachi, in collaboration between the Pathology Department and the Cardiology Department of CMH from 1st September 2021 to 28th February 2022. Laboratory-confirmed SARS-CoV-2, RT PCR positive COVID-19 cases who were admitted with complaints of chest pain with normal chest X-ray and ECG were included in the study. COVID-19 PCR-negative patients were excluded from the study irrespective of their signs and symptoms.
After getting approval from Hospital Ethical Committee (IRB No. 77/2021/TRG/ERC) and informed consent from patients or families, the confidentiality of the patients was maintained by using a coding system. The sample size was calculated using the World Health Organization (WHO) calculator keeping the prevalence of COVID-19 disease at 7.9%. Nasopharyngeal swabs were collected in viral transport media (VTM) and transported to the laboratory. SARS-COV-2-R-GENE® Real-time detection kit was used for the qualitative detection of virus RNA. A total of 104 COVID-19 RT-PCR-positive patients who were admitted to COVID-ITC with chest pain and normal chest X-ray and ECGs at the time of admission were included. Chest X-ray was done to exclude the other causes of chest pain like chest skeletal (bony) lesions, pleural effusion, pneumothorax, etc. CT scan is a gold standard for COVID pneumonia but was not done in this study because none of the patients had respiratory symptoms.
Blood samples were collected during admission for inflammatory and cardiac biomarkers including serum CRP, CK, CKMB, ALT, LDH, urea, and creatinine were collected in a gel tube and performed by photometric method on State-of-Art, fully automated chemistry analyzer Cobas C-501 by Roche diagnostics and whole blood for cTn-I, serum IL6, Ferritin, and PCT were collected in gel vacutainer tube and all were analysed by Electrochemiluminescence (ECLIA) method on fully automated endocrine analyzer Cobas e-411. Serial measurements of cTn-I and ECG were monitored as required. Groups with persistent rise of cTn-I were separated as myocarditis while other groups with the typical rise and or fall pattern of cTn-I were separated as ischemia. Clinical data were collected from COVID-ITC and COVID wards and prognosis was monitored. Discharged patients were followed till March 2022. According to the 4th Universal definition of myocardial infarction, is defined as being present when blood levels of cTn are increased above the 99th percentile upper reference limit (URL). The injury may be acute, as evident by a newly detected dynamic rising and/or falling pattern of cTn values above the 99th percentile URL, or chronic, in the setting of persistently elevated cTn levels.26 Keeping in view the above 4th universal definition, this study data were categorised into three groups based on cTn-I as normal (99th percentile = <0.01 ng/ml), mild raised, and markedly raised (>99th percentile = >0.01 ng/ml).
All the data were analysed on Statistical Package for Social Sciences (SPSS) version 25. Qualitative variables were presented as frequency and percentages. The quantitative variables like age and laboratory parameters were presented as mean and standard deviation. ANOVA test was applied to assess the severity status of myocardial injury by c-TnI level as normal, mildly raised, and markedly raised with different inflammatory/ biochemical markers. The p-value of ≤0.05 was considered statistically significant.
RESULTS
The mean age of the patients was 48 ± 16 years; 78 (75%) of them were males while 26 (25%) were females. Biochemical parameters showed a mean level of cTn-I = I.91ng/ml, C-Reactive Protein (CRP = 85mg/l), Interleukin-6 (IL-6 = 43.3), Procalcitonin (PCT = 1.40 ng/ml), Creatinine-Kinase (CK = 203 U/L), Creatinine Kinase MB (CKMB=31 U/L), and Ferritin = 471. There were only 17 (16.3%) patients who were found as renal function compromised and presented with deranged urea and creatinine (27 umol/l and 899 umol/l) respectively, while 24 (23%) had deranged ALT levels with mean ± SD, minimum and maximum raised to 72 ± 182.98, 03 and 1126 U/L, respectively (Table I). Patterns of cTn-I were analysed by serial monitoring for 6-8 hours duration and were categorised as; normal (99th percentile = <0.01 ng/ml), mild raised, and markedly raised (>99th percentile = >0.01 ng/ml), as 44 (42.4%), 38 (36.5%), and 22 (21.1%), respectively. Out of total raised cTn-I cases, persistently raised cTnI with raised inflammatory markers, 15.3% were diagnosed as cardiac injury most probably myocarditis, while 5.7% died (Figure 1).
Table I: Distribution of the demographic, laboratory, and clinical parameters among the normal (<99th percentile), raised, and markedly raised (> 99th percentile) cardiac troponin I level in patients with COVID-19.
|
Cardiac Troponin-1 Mean |
Age (years) 48+-16 |
CK (U/L) 203 |
CKMB (U/L) 31 |
CRP (mg/l) 85 |
IL6 (ng/ml) 43.3 |
PCT (ng/ml) 1.40 |
Ferritin (ng/ml) 471 |
Urea (mmol/l) 6.87 |
Creatinine (umol/l) 123 |
ALT (U/L) 71.93 |
LDH (U/L) 554 |
BNP (pg/ml) 3764.33 |
|
|
Normal (99th Percentile <0.01 ng/ml) |
Mean |
42.75 |
141.96 |
20.64 |
36.82 |
18.1988 |
.6063 |
298.50 |
5.03 |
80.88 |
37.33 |
242.29 |
474.00 |
|
N= |
44 |
44 |
44 |
44 |
44 |
44 |
44 |
44 |
44 |
44 |
14 |
2 |
|
|
Min |
23 |
29 |
12 |
1 |
1.50 |
.02 |
11 |
2 |
49 |
12 |
153 |
474 |
|
|
Max |
65 |
496 |
34 |
225 |
148.00 |
2.10 |
1370 |
14 |
127 |
143 |
499 |
800 |
|
|
Raised (>5 times of 99th Percentile) |
Mean |
50.76 |
161.00 |
23.05 |
46.95 |
20.9765 |
.6248 |
467.76 |
7.36 |
145.82 |
35.41 |
375.33 |
3179.40 |
|
N= |
38 |
38 |
38 |
38 |
38 |
38 |
38 |
38 |
38 |
38 |
18 |
10 |
|
|
Min |
26 |
35 |
12 |
0 |
6.00 |
.02 |
44 |
3 |
57 |
12 |
180 |
38 |
|
|
Max |
80 |
416 |
41 |
302 |
56.00 |
2.00 |
1989 |
27 |
899 |
67 |
823 |
9495 |
|
|
(>10 times of 99th Percentile) |
Mean |
56.91 |
401.91 |
64.15 |
247.90 |
132.7273 |
4.3627 |
852.55 |
10.10 |
179.91 |
203.85 |
1817.33 |
5836.00 |
|
N= |
22 |
22 |
22 |
22 |
22 |
22 |
22 |
22 |
22 |
22 |
6 |
6 |
|
|
Min |
30 |
72 |
14 |
0 |
3.90 |
.08 |
29 |
3 |
76 |
3 |
556 |
587 |
|
|
Max |
80 |
1599 |
242 |
521 |
691.90 |
30.90 |
2000 |
26 |
854 |
1126 |
3191 |
13454 |
|
|
Total
|
Mean |
48.37 |
203.17 |
30.63 |
84.78 |
43.3340 |
1.4070 |
471.04 |
6.87 |
123.06 |
71.93 |
554.00 |
3764.33 |
|
N= |
104 |
104 |
104 |
104 |
104 |
104 |
104 |
104 |
104 |
104 |
38 |
18 |
|
|
Min |
23 |
29 |
12 |
0.1 |
1.50 |
.02 |
11 |
2 |
49 |
3 |
153 |
38 |
|
|
Max |
80 |
1599 |
242 |
521 |
691.90 |
30.90 |
2000 |
27 |
899 |
1126 |
3191 |
13454 |
|
|
|
p= |
0.773 |
0.35 |
0.007 |
0.001 |
0.02 |
0.258 |
0.853 |
0.637 |
0.22 |
0.010 |
0.312 |
0.99 |
|
ANOVA test was applied to assess the association between the severity status of myocardial injury by cTn I with multiple biomarkers. |
|||||||||||||
Table II: Prognosis of COVID-19 assessed by of cardiac Troponin-I (n-104).
|
|
Prognosis |
n=104 |
|||
|
Recovered |
Myocarditis |
Ischemic Death |
|||
|
Cardiac Troponin -I |
Normal |
44 (42.3%) |
0 |
0 |
44 |
|
Raised |
24 (23%) |
12(11.5%) |
2(1.9%) |
38 |
|
|
Markedly raised |
14(13.4%) |
4(3.8%) |
4(3.8%) |
22 |
|
|
Total |
82(78.8%) |
16(15.3%) |
6(5.7%) |
104 |
|
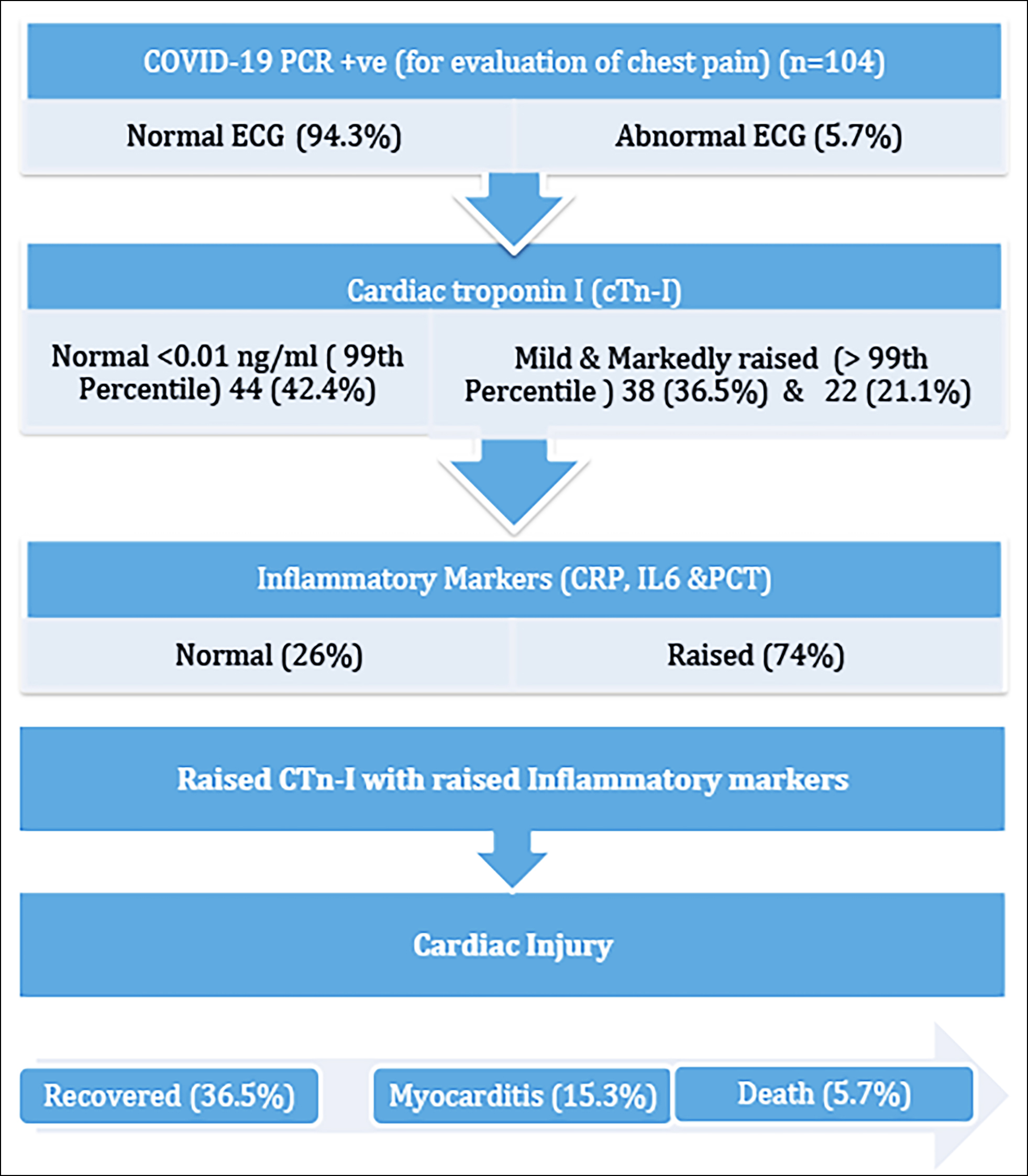 Figure 1: Association of elevated cardiac Troponin-I and inflammatory markers with COVID-19-induced cardiac injury.
Figure 1: Association of elevated cardiac Troponin-I and inflammatory markers with COVID-19-induced cardiac injury.
Relationships with other demographic, clinical, and biochemical parameters were analysed by the ANOVA test (Table I). Persistently rising pattern of cTn-I with a maximum raised to 30 ng/ml was observed in 16 patients (15.3%) who were labelled as myocarditis, while only 8 (7.6%) showed the rise and fall patterns and they all developed ischemic cardiomyopathy. Out of 104, 82 (78.8%) recovered smoothly without any adverse cardiac outcome (Table II).
Further analysis revealed that with serial monitoring of cardiac troponin, out of a total of 60 (57%, raised n= 38 and markedly raised n=22), the risk of myocarditis (persistently raised cardiac cTn-I) was found to be 15.3% more as compared to ischemia (rise and or fall pattern of troponin I) i.e. 5.7%, as shown in Table II. Depending on the clinical and biochemical parameters, out of 104, 94.3% had normal ECGs while only 5.7% developed ECG changes during admission. Unfortunately, 6 patients died due to cardiac arrhythmias and cardiac arrest secondary to COVID-induced myocardial injury and all had raised cTn-I. None of the patients were placed on ventilator support but continuous oxygen inhalation was required for those who were diagnosed with severe disease.
DISCUSSION
The emergence of SARS-CoV-2, the virus behind COVID-19, has presented a formidable challenge worldwide. Originating in Wuhan (Hubei, China), it has swiftly spread across China and numerous other countries. The resulting COVID-19 pandemic has had a profound impact on a global scale, resembling historical pandemics like the Spanish flu in its widespread effects.12 World scientists and healthcare workers (HCWs) have conducted numerous studies and trials on the disease's pathogenic causes, impacts on various body systems, and various treatment options. Respiratory involvement of the virus is the main clinical manifestation of COVID-19; however, clinical manifestations of other organ systems have also been found and documented in the vast literature published on the subject. The individuals in this study displayed respiratory involvement, as well as raised levels of urea and creatinine indicating renal issues, and elevated levels of ALT and AST suggesting hepatic involvement.
ACE-2 normally plays a favourable role in protecting tissue by being anti-inflammatory. SARS-CoV-2 infection of the cardiac tissue downregulates the expression of ACE-2 receptors which results in decreased conversion of angiotensin II to angiotensin 1-7 protein which is protective not only for lungs and heart but also for vascular endothelium, intestinal epithelium, and kidneys. It inhibits vasoconstrictor, pro-inflammatory, pro-oxidant, and pro-proliferative functions of Angiotensin II. Thus, suppression of ACE-2 receptor expression and subsequent rise in angiotensin II is the key pathogenic process involved in SARS-CoV-2 infection.13
Considering the overwhelming risk of cardiac manifestations and complications in COVID-19, it is important to investigate the cardiac status of every moderate to severe case for timely intervention and to prevent any fatality related to cardiac complications. Liaqat et al. showed that people who have critical symptoms have a much higher risk of cTn I elevation or cardiac injury than those who do not, a fact consistent with this study.13 cTn-I levels were considerably greater in individuals with severe COVID-19 infection compared to those with non-severe illness in a comprehensive evaluation of four trials involving 374 patients.14 In this study, a similar finding was also observed, with 16 patients (15.3%) showing a persistent rise in cTn-I levels, reaching a maximum of up to 30 ng/ml, indicating myocarditis. Additionally, 8 patients (7.6%) exhibited a rise and fall pattern. Patients with myocarditis had significantly higher levels of cTn-I and CRP (p <0.01). Furthermore, 6 patients (5.7%) who died due to COVID-19-induced myocardial injury had elevated cTn-I levels. In China, cardiac damage has been documented in 7% to 17% of hospitalised patients.12-16 Cardiovascular damage is more prevalent in patients admitted to intensive care units (ICUs), where it has been documented in up to 22% of cases, or in those who have died, it was up to 59%.16 Concomitant renal failure, which is frequent in severe SARS-CoV-2 infection, can raise troponin levels. A single case report highlighted a 69-year-old patient with influenza-like symptoms who rapidly progressed to respiratory distress and cardiogenic shock.11 A 35-year-old patient was hospitalised at the Institute of Mutualiste Montsouris in Paris. He had no fever or respiratory symptoms, and his sole cardiovascular risk factor was his weight (BMI = 29 Kg/m2). There was no pericardial effusion and adequate systolic function on echocardiography. The levels of cardiac Troponin I, a high-sensitivity marker, were elevated (2.885ng/ml) which is indicative of acute myocarditis. Acute myocarditis without fever, cough, or lung involvement may be a sign of COVID-19 infection, according to this case.17
The results of Lagana et al. are consistent with the results of this study.18 Similar results are reported by Tersalvi et al. in their research conducted in China.19 Similarly, elevated cTn-I levels were also found in patients with comorbidities.20 Patients with greater troponin levels, in particular, were more likely to be referred to critical care and were more likely to die as compared to normal patients.21,22
In this study, 62 patients out of 104 had significantly raised cTn-I and were admitted to ITC (59.6%); A rise in troponin, which can be used as a marker of disease severity and predicts future cardiovascular events are frequently associated with acute respiratory infections and sepsis was also evaluated by another study.23 Plasma troponin levels exhibited a substantial positive linear connection with plasma CRP levels in Guo et al.’s investigation, showing that myocardial damage may be intimately connected with inflammatory pathophysiology throughout illness progression.20 Cardiac troponin levels were also elevated in patients with community-acquired pneumonia although the aetiology is unknown, a noteworthy study conducted in the Netherlands, involving 200 individuals with 179 eligible patients, yielded a substantial dataset of 792 observation days. A total of 152 individuals (85%) had elevated troponin levels of more than 0.026 ng/ml.23 In a study done by Huang et al., 12% of COVID-19 patients were diagnosed with acute myocardial damage, which was mostly evident by increased levels of high-sensitive Troponin I.15 A similar local study showed that diabetes and hypertension were two co-morbidities that increase the chance of myocardial injury in COVID-19 patients.24
Other studies have also described a strong correlation between cTn and other inflammatory factors such as CRP with increased rates of mechanical ventilation and ventricular and atrial arrhythmias, likely due to myocardial inflammation involving the electrical circuits of myocytes. In a multicentre retrospective study of 607 COVID-19 patients in Istanbul, Turkey, laboratory results expectedly showed significant increases in the levels of CRP, PCT, and cTn-I in patients who developed ARDS and required ICU care and died.25 Ultimately, studies throughout the COVID-19 pandemic emphasise the association between cardiovascular health and disease prognosis, and thus it is critical to recognise the impact of this common comorbidity on the vulnerable population. cTn-I is a biomarker that may be used to measure myocardial injury and is regarded as a gold standard for the early detection of cardiac problems such as MI or myocarditis. Furthermore, because pre-existing cardiovascular health is a good predictor of risk and severity following SARS-CoV-2 infection, and serum cTn-I is also an independent predictor of COVID-19 disease severity and mortality, the function of cTn-I in COVID-19 risk stratification is crucial to explore. The goal of this study is to provide an updated perspective on the clinical importance of cTn-I in COVID-19.
This research has some limitations, such that this is a single-centre experience. Furthermore, because of the short study period and the time constraints brought on by the disease's ongoing peak period, echocardiographic changes were also not studied. Further research with a larger sample size and given a thorough evaluation by monitoring more parameters with better modalities might provide a more comprehensive picture of cardiac involvement.
CONCLUSION
Cardiac Troponin I levels have proved to be a more effective biomarker for detecting myocardial damage in COVID-19 patients and can be used as an independent predictor for the severity of cardiac injury and death. They have been shown to have a better predictive value for at-risk patients than other inflammatory biomarkers. In COVID patients, greater levels of cTn-I have been linked to a higher risk of CVD complications and death. The authors recommend that levels of cTn-I should be checked in every serious COVID-19 patient to exclude cardiac involvement.
ETHICAL APPROVAL:
Ethical approval for this research was obtained from the Ethical Committee (IRB No. 77/2021/TRG/ERC) prior to the initiation of this research.
PATIENTS’ CONSENT:
Informed consent was obtained from the patients and/or their families/legal guardians to publish the data concerning their cases prior to publishing the data, the confidentiality of the patients was maintained by using the coding system.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
AR: Contributed to data collection, drafted the introduction and discussion, conducted the related literature search, and performed reference settings.
SY: Contributed to the material and methods section, interpreted the data, and conducted the statistical analysis.
GRM: Contributed to the conception and study design, provided critical evaluation, and contributed to the conclusion.
SRAN: Contributed to the conception and design of the work.
GM: Contributed to the discussion section.
AA: Provided critical evaluation of the work.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Imazio M, Klingel K, Kindermann I, Brucato A, Francesco R, Adler Y, et al. COVID-19 pandemic and troponin: Indirect myocardial injury, myocardial inflammation, or myocarditis? Heart 2020; 106(15):1127. doi: 10.1136/heartjnl-2020- 317186.
- South AM, Diz DI, Chappell MC. COVID19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 2020; 318(5):H1084-90. doi: 10.1152/ajpheart. 00217.2020.
- Significance of cardiac troponins as an identification tool in COVID-19 patients using biosensors: An Update. Front Mol Biosci 2022; 9:821155. doi: 10.3389/fmolb.2022.821155.
- Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KN, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020; 323(20):2052-9. doi: 10.1001/jama.2020.6775. Erratum in: JAMA 2020; 323(20):2098.
- Metkus TS, Sokoll LJ, Barth AS, Czarny MJ, Hays AG, Lowenstein CJ, et al. Myocardial injury in severe COVID19 compared with non COVID19 acute respiratory distress syndrome. Circulation 20201; 143(6):553-65. doi: 10.1161/CIRCULATIONAHA.120.050543.
- Sandoval Y, Jr JL, Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID19: JACC review topic of the week. J Am Coll Cardiol 2020 Sep; 76(10):1244-58. doi: 10.1016/j.jacc.2020.06.068.
- Lara D, Young T, Toro D, Chan V, Ianiro C, Hunt K, et al. Acute fulminant myocarditis in a pediatric patient with COVID19 infection. Pediatrics 2020; 146(2):e20201509. doi: 10.1542/peds.2020-1509.
- Alhogbani T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann Saudi Med 2016; 36(1):78-80. doi: 10.5144/0256-4947.2016.78.
- Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, et al. Acute myocarditis presenting as a reverse TakoTsubo syndrome in a patient with SARSCoV2 respiratory infection. Eur Heart J 2020; 41(19):1861-2. doi: 10. 1093/eurheartj/ehaa286.
- Chen C, Chen C, Yan J, Zhou N, Zhao J, Wang D. Analysis of myocardial injury in patients with COVID19 and association between concomitant cardiovascular diseases and severity of COVID19. Zhonghua Xin Xue Guan Bing Za Zhi 2020; 48(7):567-71.
- Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID19 cardiogenic shock. Eur J Heart Fail 2020; 22(5):911-5. doi: 10.1002/ejhf.1828.
- Patterson GE, Marie MK, Clough HE, Rushton J. Societal impacts of pandemics: Comparing COVID19 with history to focus our response. Front Public Health 2021; 9:630449-9. doi: 10.3389/fpubh.2021.630449.
- Liaqat A, AliKhan RS, Asad M, Rafique Z. Evaluation of myocardial injury patterns and ST changes among critical and noncritical patients with coronavirus19 disease. Sci Rep 2021; 11(1):4828-8. doi: 10.1038/s41598-021-84467-4.
- Lippi G, Lavie CJ, SanchisGomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID19): Evidence from a metaanalysis. Prog Cardiovas Dis 2020; 63(3):390-1. doi: 10.1016/j.pcad.2020.03.001.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395(10223):497-506. doi: 10.1016/S0140-6736(20)30183-5.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395(10229):1054-62. doi: 10.1016/S0140- 6736(20)30566-3.
- Paul J, Charles P, Richaud C, Caussin C, Diakov C. Myocarditis revealing COVID19 infection in a young patient. Eur Heart J Cardiovasc Imaging 2020; 21(7):776-6. doi: 10.1093/ehjci/jeaa107.
- Laganà N, Cei M, Evangelista I, Cerutti S, Colombo A, Conte L, et al. Suspected myocarditis in patients with COVID19: A multicentre case series. Medicine. 2021; 100(8):e24552-2. doi: 10.1097/MD.0000000000024552.
- Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D. Elevated troponin in patients with coronavirus disease 2019: Possible mechanisms. J Cardiac Fail 2020; 26(6):470-5. doi: 10.1016/j.cardfail.2020.04.009
- Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID19). JAMA Cardiol 2020; 5(7):811-8. doi: 10.1001/jamacardio.2020.1017.
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalised patients with 2019 novel coronavirus infected pneumonia in Wuhan, China. JAMA 2020; 323(11):1061-9. doi: 10.1001/jama.2020.1585.
- Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020; 46(5):846-8. doi: 10.1007/s00134-020-05991-x.
- Frencken, Jos F, Baal, Kappen TH, Donker DW, Horn J, van, et al. Myocardial injury in critically ill patients with communityacquired. Ann Am Thorac Soc 2019; 16(5):606612. doi: 10.1513/AnnalsATS.201804-286OC.
- Ali J, Khan FR, Ullah R, Hassan Z, Khattak S, Lakhta G, et al. Cardiac troponin I levels in hospitalised COVID19 patients as a predictor of severity and outcome: A retrospective cohort study. Cureus 2021; 13(3):e14061-1. doi: 10.7759/cureus. 14061.
- Barman HA, Atici A, Sahin I, Alici G, Tekin A, Baycan, Omer Faruk, et al. Prognostic significance of cardiac injury in COVID19 patients with and without coronary artery disease. Coron Artery Dis 2021; 32(5):359-66. doi: 10.1097/MCA. 0000000000000914.
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction. Circ 2018; 138(20): 138:e618-e651. doi/10.1161/CIR. 0000000000000617.