Breastfeeding/Breast Milk Safety in Infants of Mothers with Suspected or Confirmed COVID-19 Infection
By Nursan Cinar1, Ozge Karakaya Suzan1, Sinem Ozturkler2, Vedat Cinar2, Pinar Tabakoglu2Affiliations
doi: 10.29271/jcpsp.2022.04.492ABSTRACT
The purpose of this systematic review was to determine whether suspected or confirmed COVID-19 infected mothers in the lactation period can breastfeed their infants; and whether suspected or confirmed COVID-19 infected mothers can breastfeed their infants by taking some precautions. The study also aimed to present the measures that can be taken in line with the evidence. The studies conducted after November 2019 and including infants of suspected or confirmed COVID-19 infected mothers were reviewed between 2019 and 2020. A literature review was conducted in five electronic databases (MEDLINE, PubMed, ScienceDirect, Web of Science, and Scopus) to reach original quantitative studies in English. The present authors retrieved 46 of the 1,229 studies included after screening. Three studies were cross-sectional studies, 30 were case studies, and 13 were cohorts. In most of the sources examined, it was concluded that most of the breastfed infants had negative findings for SARS-Cov-2 infection in PCR. In line with the limited data available, it was concluded that most of the breastfed infants had negative findings for SARS-Cov-2 infection in PCR; and breastfeeding mothers should initiate breastfeeding by taking protective measures.
Key Words: Breastfeeding, COVID-19, Suspected COVID-19 infected mothers, Confirmed COVID-19 infected mothers.
INTRODUCTION
Coronavirus disease 2019 (COVID-19), also named SARS-CoV-2, is a respiratory tract infection caused by a newly emerging coronavirus in Wuhan, China, in December 2019. SARS-CoV-2 or COVID-19 infection was most commonly observed in the 30-79 years age range.1 While the epidemic was taken under control in China as a result of taking strict isolation and quarantine measures at the end of three months, the number of COVID-19 cases increased in many countries. In March 2020, the World Health Organization declared it a pandemic. According to COVID-19 data, it caused 167,252,150 cases of infection and 3,467,663 deaths worldwide as of May 25, 2021.2-4
A study by Huang et al. reported epidemiological, clinical, laboratory, and radiological features and treatment and clinical outcomes of patients with COVID-19 pneumonia.5 However, it primarily covered non-pregnant adults.
The clinical features of COVID-19 pneumonia in pregnant women, neonates, and children were not reported. The number of pregnant women and neonates with COVID-19 is also increasing,6 which leaves many question marks about feeding and breastfeeding newborns during pregnancy and the postpartum period. At the same time, the epidemic started, mothers who continued breastfeeding experienced uneasiness and needed reliable advice in this regard. It is very important to protect and maintain breastfeeding during the outbreak because breast milk has unique nutritional properties and immune-boosting and infection-protecting properties. Bioactive immunological factors in breast milk are important for the development of the neonate’s immune system. It has been proven that these biologically active elements protect the neonate from infections and play a significant role in the maturing of the immune system. They have an especially strong protective impact against infectious diseases prevented through the direct transfer of antibodies and other anti-infective factors; and the long-term transfer of immunological sufficiency and memory.2 At the same time, if a mother or infant is infected, the immunoreactive biochemical factors of breast milk change. The breast milk has more active immune milk content.
Transmission through breast milk has not been reported in infants.7,8 In the study performed by a group of Chinese experts at the beginning of February 2020, the infant of a suspected or confirmed COVID-19 infected mother was not breastfed. Wang et al. reported that COVID-19 suspected or diagnosed mothers’ infants should be fed with breast milk if their breast milk tests are negative.9 On the contrary, no concrete data support this suggestion and show that COVID-19 is transmitted through breast milk. In a recent study carried out by Chen et al., six of nine pregnant women diagnosed with COVID-19 in the laboratory in the last trimester, had negative breast milk, throat swabs, amniotic fluid and cord blood samples taken after cesarean delivery and the results of tests for SARS-CoV-2, and three mothers reported that testing could not be performed because no sample could be taken.8
It is important whether suspected or confirmed COVID-19 infected mothers during the lactation period can breastfeed their infants and whether suspected or confirmed COVID-19 infected mothers can breastfeed their infants by taking some precautions. The measures that can be taken also need to be evidence-based.
The aim of this systematic review was to examine the safety of breastfeeding or feeding infants with breast milk by suspected or confirmed COVID-19 infected mothers.
METHODOLOGY
A review protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD420 20179512; https://www.crd.york.ac.uk/prospero/display_record. php?RecordID=179512). The PRISMA and flow diagram was created. The studies conducted after November 2019 and investigating breastfeeding/breastfeeding safety in infants of suspected or confirmed COVID-19 infected mothers were reviewed from March to November 2020.
The question of this systematic review was formed using the PICO criteria.
P Stood for population, which included suspected or confirmed COVID-19 infected mothers in the lactation period and their infants. There were no restrictions on age or ethnicity.
I Intervention – "Breastfeeding," "feeding with breast milk," and "preventive measures while breastfeeding" in suspected or confirmed COVID-19 infected mothers.
C Comparison – There will be no limitation on the type of comparator.
O Outcome – Breast milk intake/breastfeeding safety.
The primary outcome criterion was an infant with confirmed SARS-CoV-2 infection during breastfeeding or receiving expressed breast milk from a woman with suspected or confirmed SARS-CoV-2 infection. The secondary outcome criterion was infection outcomes of the infants receiving breast milk analysed for SARS-CoV-2 infection. The selection criteria are summarised in Table I.
Medical Subjects Headings (MeSH) for English keywords and the content of Turkiye Bilim Terimleri (TBT, https://www.bilimteri mleri.com/) were used to create Turkish equivalents of the English keywords. A review was made according to the keywords specified for screening in MEDLINE, Web of Science, PubMed, ScienceDirect, and Scopus databases and the keywords of "Breastfeeding" (Breastmilk OR Human milk OR Lactation) and "COVID-19" (suspected with COVID-19 infected mothers OR confirmed, COVID-19 infected mothers OR infected mother with COVID-19, Coronavirus OR Sars-Cov2).
The process of study selection was conducted following the PRISMA flow diagram in three steps. These steps include the evaluation of the title, summary, and full texts of the studies. First of all, the studies were evaluated in terms of title convenience by the researchers (OKS, SO, VC, PT), who also scanned the databases. The studies that did not meet the criteria for title inclusion were eliminated at that stage. Afterward, the five independent researchers evaluated the summaries in terms of inclusion criteria. As a result of the evaluation, a consensus was reached between the five researchers (NC, OKS, SO, VC, PT). The studies that met the inclusion criteria for abstract were recorded using the EndNote X9 program, and their full texts were downloaded. The screening process was recorded in the PRISMA flow diagram (Figure 1).
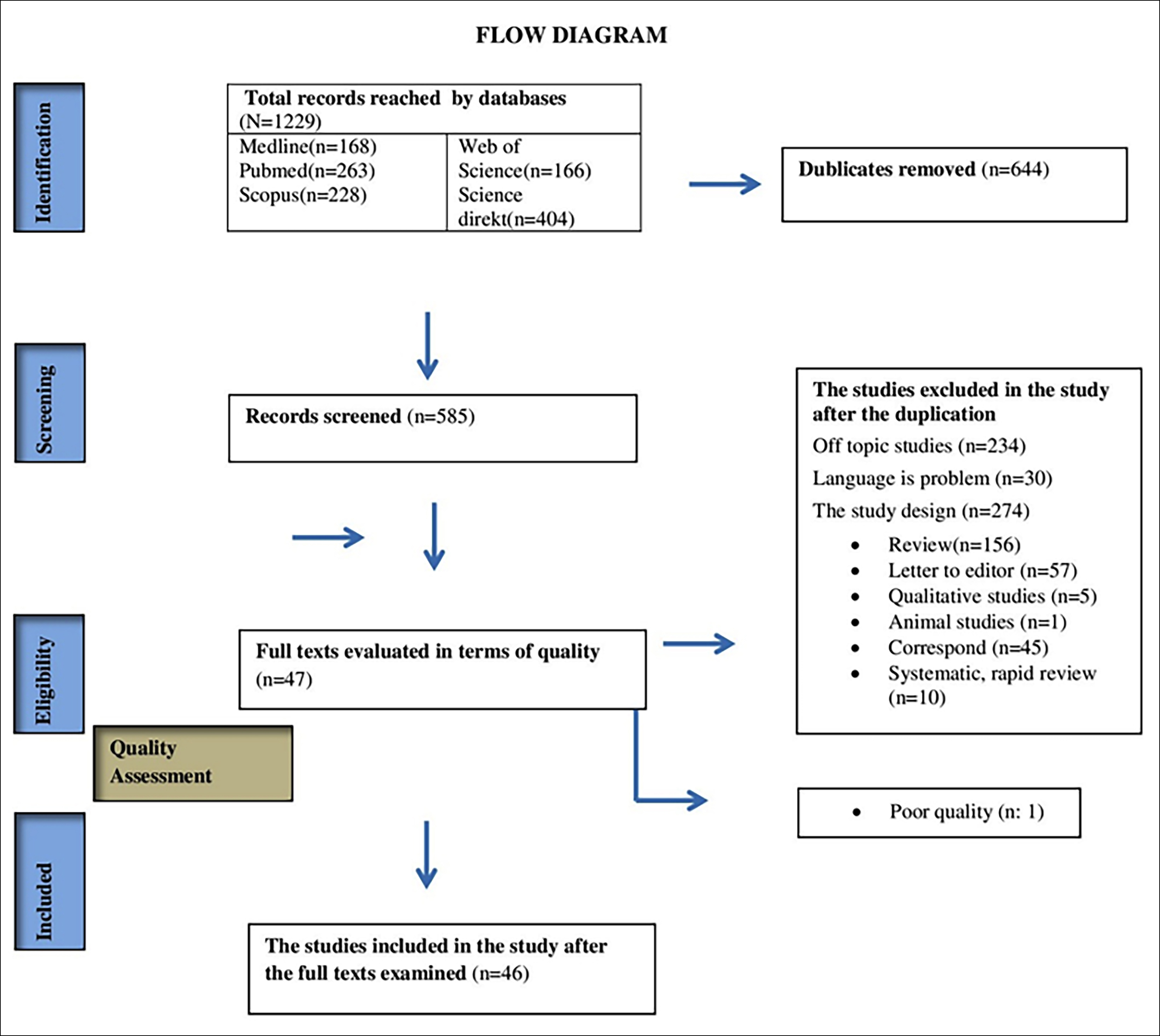 Figure 1: The flow chart (flow diagram) for the selection of studies.
Figure 1: The flow chart (flow diagram) for the selection of studies.
The first and second researchers (NC, OKS) performed the extraction of data on trial features, methodology, participant features, intervention features, outcome measures, and outcome data independently, and the other researchers checked them. The studies that were decided to be included in the study by both researchers were included in the review. The studies on which no consensus could be reached were reviewed by both researchers again (NC, OKS). The studies that were considered not meeting the inclusion criteria by the two researchers (NC, OKS) were excluded. The data, including data on authors, year of publication, definition of participants, research methods, and results, were extracted.
The coding table of the studies included in the systematic review contains information on the study type, design/sample size, the purpose of the study, data collection tool, study findings, and recommendations.
Two independent researchers performed quality assessments for every study. The QATQS was utilised for quantitative research. The QATQS was employed to assess the selection bias of the studies, study design, confounders, blinding, data collection method, and exclusion/abandonment. Thomas et al. performed the validity and reliability studies of the original scale,10 while Ergin and Akın carried out the validity and reliability studies of the Turkish version of the tool (T-QATQS).11 Using the said tool, it is possible to classify the methodological quality of the studies as weak, medium, and strong.
To use QATQS in the research, written permission was obtained for performing the validity and reliability studies of the tool in Turkish. No funding sources were utilised in the current study.
RESULTS
One thousand two hundred and twenty-nine studies were acquired in the screening performed in five databases. Six hundred forty-four were excluded due to duplication. After excluding the duplications, a further 538 studies were excluded since they were published in a language besides English and Turkish, were unrelated to the subject (n = 234), or were inappropriate for the study design. Two independent researchers reviewed the full texts of the remaining forty-seven studies in terms of suitability and quality. A study was excluded since it obtained a low score. Thirty of the remaining 46 studies were case reports and were directly submitted to the study. The remaining 16 studies were assessed using QATQS. In the assessment, 16 studies of medium quality were included in the systematic review.8,12-26 Figure 1 presents the flow diagram for the selection of studies.
Sixteen of the 46 studies, included in the present systematic review, were research articles. The populations of the assessed studies consisted of suspected or confirmed COVID-19 infected mothers during the lactation period. The sample size varied between one and 101. Studies were cross-sectional 8,12,24 case studies,27-56 retrospective studies,13,16,20 descriptive studies,22 and cohort studies.14,15,17-19,23,25,26 The mentioned report articles were included to conduct narrative analysis.
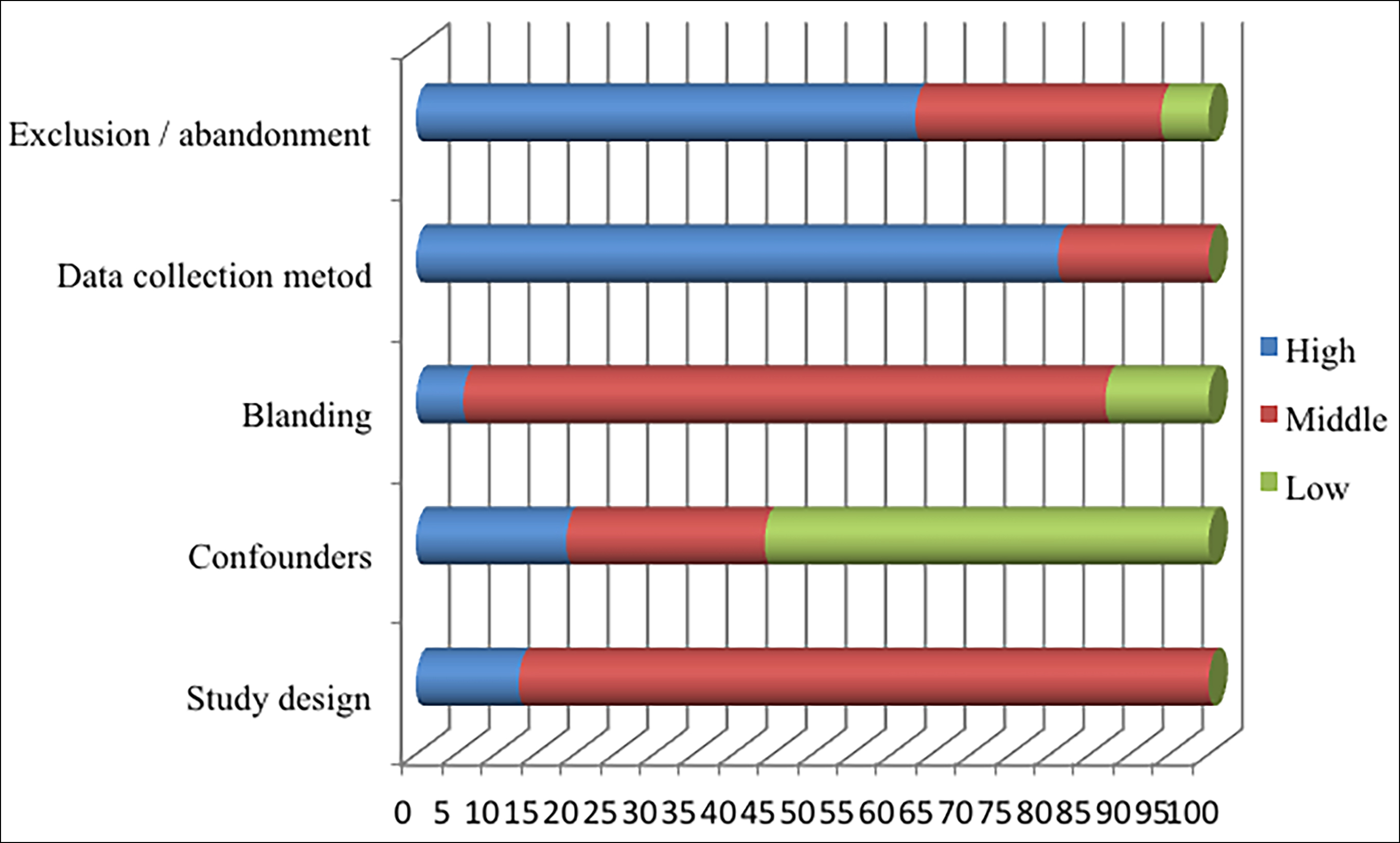 Figure 2: Graph showing the distribution of the scores received from each field, according to the QATQS of all studies assessed in terms of quality (n = 16).
Figure 2: Graph showing the distribution of the scores received from each field, according to the QATQS of all studies assessed in terms of quality (n = 16).
The score distribution of the 16 studies in the systematic review, according to the QATQS, is presented in Figure 2. Among case report studies, studies with laboratory findings were taken directly (30 studies).
Table II demonstrates an outline of the outcomes in every study reviewed. The said outcomes were analysed and reviewed thematically.
The primary outcome criterion was an infant with confirmed SARS-CoV-2 infection receiving expressed breast milk or breastfed by a woman with suspected or confirmed SARS-CoV-2 infection (Table II and Figure 3). According to the findings of the studies that performed the SARS-CoV-2 examination in neonates born to mothers diagnosed with COVID-19, breast milk samples were examined using a diagnostic tool such as RT-PCR and chest radiography, and laboratory findings were analysed. Among the breast milk samples examined, while SARS-CoV-2 was not detected in breast milk in the studies of 8,13, 15,26,27,30,31,34,36,38,39,41,43,45,49,50,52,54,56 SARS-CoV-2 was detected in breast milk in the studies of .26,29,40,41,46,51,56
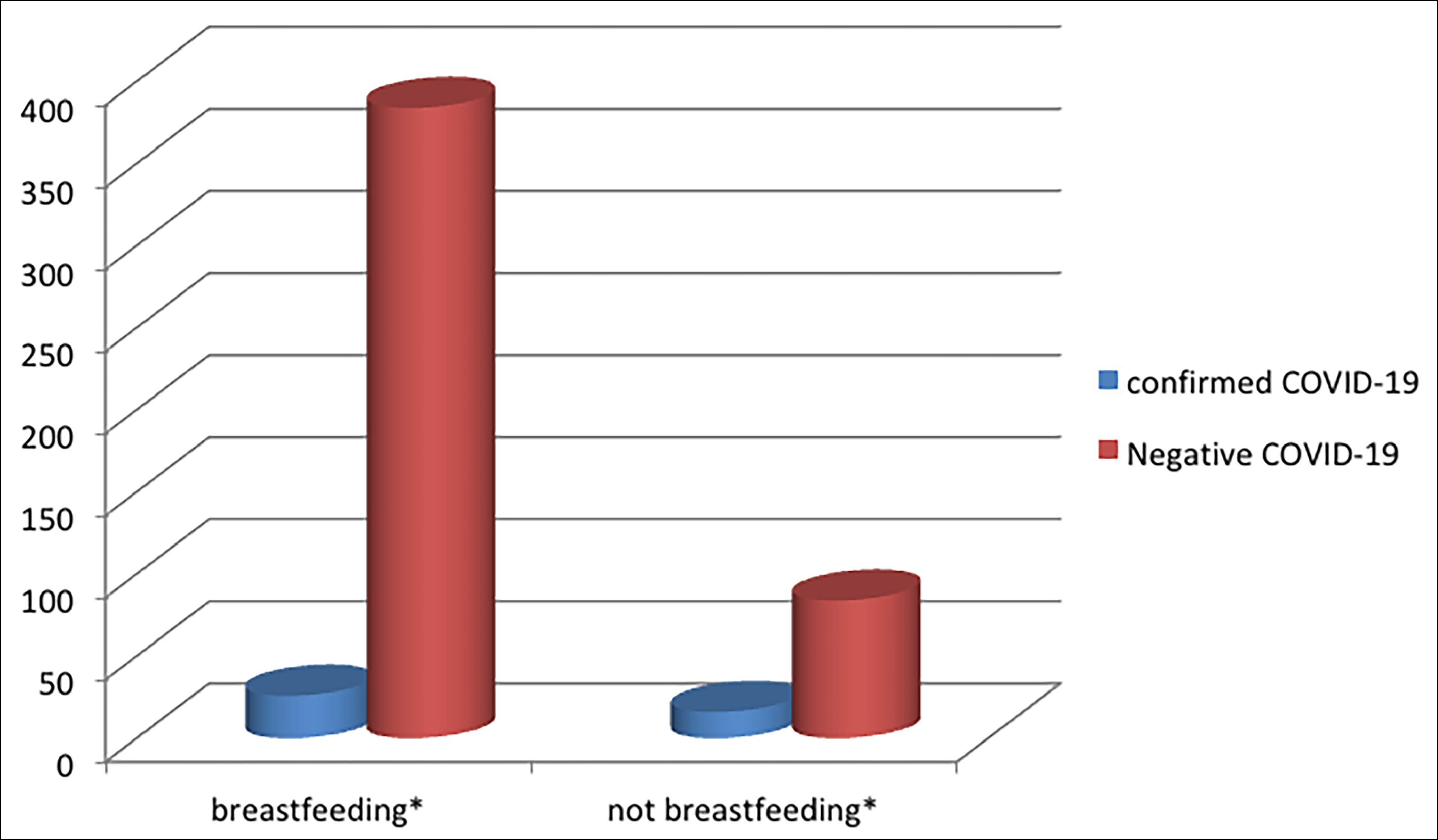 Figure 3: Presence of COVID-19 in breastfed and non-breastfed infants (n = 510).
Figure 3: Presence of COVID-19 in breastfed and non-breastfed infants (n = 510).
*Babies who were definitely stated to be breastfed or not breastfed were included in the chart.
Table I: Eligibility criteria.
|
Inclusion criteria |
Exclusion criteria |
|
Mothers with suspected or confirmed COVID-19 infection and their infants |
Mothers with probable COVID-19 infection |
|
Studies with mothers aged between 18-45 years during lactation |
Mothers aged below 18 and above 45 years during lactation |
|
Mothers with suspected or confirmed COVID-19 infection cared for at home or in clinics |
Mothers with confirmed COVID-19 infection in the intensive care unit |
|
Randomized Controlled Studies Semi-experimental studies
Descriptive, cross-sectional, case-control and case reports
|
Expert opinions Qualitative studies Unpublished theses Summary studies In vitro or animal study Systematic and rapid reviews |
|
Studies published in Turkish and English
|
Studies published in languages besides Turkish and English |
|
Published after 1 December 2019 |
|
Table II: Coding table of the studies included in the systematic review (N=46).
|
Authors/* Year |
Types of studies |
Design/sample size N* |
Aim of the study |
Data collection tool |
Where the test sample was taken |
Study results |
Suggestions |
Studies Quality Assessment |
|
Chen et al. (2020b)8 |
Research Article |
Cross-sectional / n:9 |
“Evaluated the clinical characteristics of COVID-19 in pregnancy and the intrauterine vertical transmission potential of COVID-19 infection.” |
qRT-PCR |
throat swab breastmilk samples |
“Breastmilk samples from six patients were tested for SARS-CoV-2, and all samples tested negative for the virus.” |
|
Middle |
|
Ferrazzi et al. (2020)12 |
Research Article |
Cross-sectional /n:42 |
“To report mode of delivery and immediate neonatal outcome in COVID-19 infected women.” |
RT-PCR |
nasopharyngeal swab |
“Reports the obstetric outcome of a cohort of COVID-19 affected pregnant women and the rate of SARS-CoV-2 positivity in newborns according to the mode of delivery and breastfeeding status.” |
“Use mask and safe procedures in all breastfeeding mothers.” |
Middle |
|
Gao et al. (2020)13 |
Research article |
Retrospectively/ N:14 |
“It was assessed the clinical and immunologic features of COVID-19–affected mother–infant pairs; specifically tested breast milk for pathogens, SARS-CoV-2 neutralizing antibodies and immunologic components; and explored the feasibility of breastfeeding and related transmission possibilities.” |
PCR (n:14) |
oropharyngeal or nasopharyngeal swabs, breast milk sample, meconium sample |
“In the findings, SARS-CoV-2 was not detected in breast milk, and the mothers who continued breastfeeding had no breast lesions” (n:12) “four patients continued breastfeeding while taking precautions.” |
“Breastfeeding has a low risk of transmitting SARS-CoV-2 or escalating maternal disease; mothers should continue to breastfeed but should take prudent precautions. Infants can additionally benefit from direct acquisition of antibodies against SARS-CoV-2 through breast milk.” |
Medium |
|
Savasi et al. (2020)14 |
Research article |
Cohort study/N:57 |
“To investigate the clinical evolution of coronavirus disease 2019 (COVID-19) in hospitalized pregnant women and potential factors associated with severe maternal outcomes.” |
PCR |
nasopharyngeal swab |
“For all these newborns, rooming-in and breastfeeding were performed.” “Four of 57 were diagnosed with SARS-CoV-2 infection in the early postpartum period.” |
|
Medium |
|
Sahin et al. (2020)15 |
Research article |
Cohort Study/N:29 |
“To evaluate the course and effect of coronavirus disease 2019 (COVID‐19) on pregnant women followed up in a Turkish institution.” |
PCR |
nasopharyngeal and oropharyngeal breast milk samples |
“None of the neonates were positive for SARS‐CoV‐2.” “Samples of breastmilk were also negative for the virus.” |
|
Medium |
|
Hirshberg et al. (2020)16 |
Research article |
Retrospective Review/ N:18 |
“Records related to neonates born to COVID-19 positive women were also reviewed.” |
PCR |
Nasopharyngeal swab |
“17 infants tested negative, the remaining infant had an “indeterminant” test result.” “Breastfeeding was encouraged with hand hygiene and maternal masking.” |
|
Medium |
|
Salvatore et al. (2020)17 |
Research article |
Cohort Study/N:82 |
“It was aimed to elucidate best practices regarding infection control in mother–newborn dyads, and identify potential risk factors associated with transmission.” |
PCR |
nasopharyngeal swabs |
“All neonates were tested at 24 h of life and none were positive for SARS-CoV-2.” “All mothers were allowed to breastfeed; at 5–7 days of life, 64 were still breastfeeding.” |
“The data suggest direct breastfeeding are safe procedures when paired with effective parental education of infant protective strategies.” |
Medium |
|
Griffin et al. (2020)18 |
Research article |
Cohort study/ N=62 |
The objectives of the study are to describe the effects of COVID-19 on the obstetric and neonatal population in an area with a high incidence of SAR-CoV-2 infection. |
RT-PCR |
nasopharyngeal swab |
“Fifteen infants had their SARS-CoV-2 RT-PCR testing performed from a nasopharyngeal swab taken after 24 hours of life. Most (14.93%) were performed in neonates whose mothers were confirmed COVID-19 positive. Fourteen of the tests were negative, one was insufficient for analysis. No infant tested positive for COVID-19.” |
“Human milk feeds were encouraged and mothers were provided electronic breast pumps, and lactation support to provide mother’s own milk that could be fed to the infant during their period of isolation, and to allow direct breastfeeding once the infant and/or mother was cleared from isolation.” |
Medium |
|
Cojocaru et al. (2020)19 |
Research article |
Cohort study /N=17 |
“This quality improvement project was conducted to determine whether maternal bonding is safe for neonates whose mothers tested positive for SARS-CoV-2.”
|
PCR |
oropharynx and nasopharynx swab |
“All neonatal tests for SARS-CoV-2 returned negative.” “16 fed the infants with breastmilk (11 direct breastfeedings and five pumped the breast milk).” |
|
Medium |
|
Anand et al. (2020)20 |
Research article |
Retrospectively N=65 |
“The study was conducted to describe the clinical profile of neonates born to mothers who tested positive for COVID 19 infection and to determine the association of neonatal COVID 19 status and viral load with maternal clinical status and viral load.” |
RTPCR |
nasopharyngeal/oropharyngeal swabs |
“Of 65 tested neonates, 7 were confirmed COVID 19 positive by RTPCR.” “It is reassuring that despite most neonates being roomed-in with mother and breastfed since birth (51 of the 65), only 4 neonates tested positive.” |
“The unit followed the policy of rooming in with mother and direct breastfeeding with precautions in stable neonates born to COVID 19 positive mothers.” |
Medium |
|
Pereira et al. (2020a)21 |
Research article |
Cohort study N=23 |
“The aim of study was to describe experience in the clinical management of 60 COVID-19-positive pregnant women who were attended to in the hospital during the first month of the epidemic in Spain.” |
PCR |
nasopharyngeal swabs |
“In all of cases, neonates tested negative.” “No vertical or horizontal transmissions were diagnosed in the neonates during labor or breastfeeding.” |
|
Medium |
|
Marín Gabriel et al. (2020a)22 |
Research article |
Descriptive study /N=42 |
“The aim was to describe the clinical features of mothers infected with COVID-19 and examine any potential vertical mother to newborn transmission.” |
PCR |
nasopharyngeal swab oropharyngeal swab |
“All other tests on the infants negative.”
“19 babies were breastfed.” |
“it is essential to keep the mother and child together, when clinically possible, and promote, and guarantee, correct breastfeeding support during their hospital stay and when they are discharged.” |
Medium |
|
Farghaly et al. (2020)23 |
Research article |
Cohort study/N:15 |
“This study aims to assess the characteristics of newborns born to SARS-CoV-2-positive women compared with those mothers who tested negative.” |
PCR |
nasopharyngeal swab |
“It had only one newborn who tested positive for SARS-CoV-2.” “Five newborns of positive mothers directly breastfed with precautions.” |
|
Medium |
|
Patil et al. (2020)24 |
Research article |
Retrospective Cross-Sectional Study/N:45 |
“It is aimed to describe the unique experience from Baby Friendly hospital at the epicenter of the COVID19 pandemic.” |
PCR |
oropharyngeal and nasopharyngeal swab |
“A total of 33 newborns colocated with their mothers and 31 of the 33 were breastfed with the initiation of breastfeeding within 1 h of birth.” “A total of 42 newborns tested negative and 3 tested positive.” |
|
Medium |
|
Dumitriu et al. (2020)25 |
Research article |
Cohort Study/N:101 |
“To describe the outcomes of neonates born to mothers with perinatal SARS-CoV-2 infection and the IP&C practices associated with these outcomes.” |
PCR |
nasopharyngeal swab |
“Most mothers 91 breastfed at least partially.” “101 neonates born to mothers with perinatal SARS-CoV-2 infections, 2 (2.0%) had positive test results for SARS-CoV-2.” |
|
Medium |
|
Wu et al. (2020b)26 |
Research article |
Cohort Study/N:5 |
“To assess whether vaginal secretions and breast milk of women with coronavirus disease 2019 (COVID-19) contain severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).” |
PCR |
neonatal throat and anal swabs. Breast milk sample |
“SARS-CoV-2 nucleic acid tests of neonatal throat and anal swabs were all negative on the 1st and 3rd days after birth.” “It is assessed breast milk in three women; the breast-milk sample of one woman (patient 5), collected on the 1st day after delivery, was positive using the PCR.” |
Mothers with COVID-19 should not breastfeed until after full recovery, when breast milk tests negative for the virus.” |
Medium |
|
Li et al. (2020)27
|
Case Study |
N:1 |
“Examination of newborn born from mother diagnosed with COVID-19.” |
- |
a sputum sample oropharyngeal swab specimen breast milk sample |
“Breast milk samples were negative. Mother-to-child transmission is unlikely for this virus.” |
“Effective implementation of protection measures during delivery, including a negative-pressure delivery room, may help prevent the infant from acquiring SARS-CoV-2 infection.” |
|
|
Lowe & Bopp (2020)28
|
Case Report |
N=1 |
“Describing an uncomplicated vaginal birth in a SARS-CoV-2 positive mother.” |
PCR |
nasopharyngeal (NP) swabs |
“Neonatal COVID- 19 testing was performed at 24 hours post delivery which was negative.” “The neonate was breastfed throughout.” |
“That it is possible to consider rooming in post delivery for COVID-19 positive parents. Encouragement of breastfeeding appears possible and safe when viral precautions are observed.” |
|
|
Yu et al. (2020)29 |
Case Report |
N=1 |
“To assess the safety of breastfeeding and the possible protective effects of breast milk on infants, the presence of SARS-CoV-2 nucleic acid was determined in maternal serum, breast milk, nasopharyngeal (NP) swabs and feces, and in the infant’s serum, NP swabs and feces.” |
PCR |
maternal serum, breast milk, nasopharyngeal (NP) swabs and feces, and in the infant’s serum, NP swabs and feces |
“the patient’s milk was positive for SARS-CoV-2 IgG and negative for IgM. On hospital day 14, the child’s serum was positive for both SARS-CoV-2 IgG and IgM, suggesting either breastmilk transfer to the infant or infant de novo production of the IgG and/or IgM, or both mechanisms. A protective antibody can be passed by breastfeeding from the mother to the offspring, preventing or decreasing the severity the children’s diseases.” |
“Observational studies suggest that breast milk is safe. Future studies should address the issue of detecting the level of SARS-CoV-2 IgG to assess the best time window for breastfeeding, and the risks vs benefits of direct breastfeeding.” |
|
|
Kam et al. (2020)30 |
Case report |
N=1 |
“Understanding the clinical manifestation of COVID-19 across all population groups, especially infants and young children, is critical for public health containment measures to be effective.” |
PCR |
nasopharyngeal swabs, breast milk samples |
“nasopharyngeal swabs continued to be positive for SARS-CoV-2.” “Breast milk samples on day 11 of illness were negative for SARS-CoV-2.” |
|
|
|
Wang et al. (2020b)31 |
Case report |
N=1 |
“Whether COVID-19 has mother-to-child vertical transmission, and its short-term and long-term harm to offspring, is still unclear.” |
PCR |
pharyngeal swab breast milk sample
|
“The result of pharyngeal swab for SARS-CoV-2 was positive at 36 hours after birth.” “The mother’s breast milk sample was negative for SARS-CoV-2 as well.” |
“It was recommended the mother not to breastfeed and to empty the breast milk to avoid mastitis.” |
|
|
Lebrão et al. (2020)32 |
Case report |
N=1 |
|
PCR |
nasopharyngeal swab |
“The participant newborn tested negative in one nasopharyngeal swab RT-PCR performed within 48 hr of life.” |
“The participant was instructed by the nursing team to wear surgical masks, especially when she was in contact with her newborn infant, and to wash her hands or use hand sanitizer before and after touching her baby or directly breastfeeding.” |
|
|
Perrone et al. (2020a)33 |
Case report |
N=4 |
It herein reports a series of four neonates whose mothers had recovered from new coronavirus 2019 disease (COVID-19) diagnosed in the third trimester of pregnancy |
PCR |
Nasopharyngeal swab |
“The participant newborn tested negative in one nasopharyngeal swab RT-PCR.” “The mothers breastfed the neonates.”
|
|
|
|
Dong et al. (2020a)34 |
Case report |
N=1 |
“It was followed up the viral loads and antibody titers of SARS-Cov-2 for a mother and the neonate since hospitalized to discharged.” |
PCR |
oropharyngeal swab breast milk sample |
“The infant’s An oropharyngeal swab specimen, obtained immediately after she was taken from the uterus, showed a negative result for the detection of SARS-CoV-2 RNA.” “Breast milk, collected were negative.” |
“More importantly, the IgG and IgA antibodies were detected in breast milk indicate the potential immune protection for the neonates.” |
|
|
Al-Kuraishy et al. (2020)35 |
Case report |
N=1 |
“This study presents clinical feature and management of pregnant women with confirmed COVID-19 pneumonia with suspected vertical transmission to premature baby.” |
PCR |
Antigen-antibody reactions |
“The neonate was referred COVID-19 PCR test. The result was negative, and the mother started breastfeeding of her baby and was discharged home.” |
|
|
|
Marín Gabriel et al. (2020b)36 |
Case report |
N:7 |
“The objective of our study was to determine whether the SARS-CoV-2–positive mothers transmit the virus to their hand-expressed colostrum.” |
PCR |
nasopharyngeal swab breast milk sample |
“SARS-CoV- 2 was not detected in any of the colostrum samples obtained in our study.” “All other tests on the infants negative.” |
“Hand expression (assuring that a mask is used and that appropriate hygienic measures are used for the hands and the breast), when direct breastfeeding is not possible, appears to be a safe way of feeding newborns of mothers with COVID-19.” |
|
|
Chhabra et al. (2020)37 |
Case report |
N:1 |
“It describes the anaesthetic management of a mildly symptomatic, COVID‑19 positive, 28‑year‑old second gravida with term pregnancy who was taken up for an elective caesarean section under subarachnoid block in a standalone maternity facility.” |
PCR |
- |
“The baby was breastfed.” “The baby was tested on day 10 and 14 after birth using reverse transcriptase–PCR and was negative both times.” |
“She was instructed to keep on the N‑95 respirator and maintain hand hygiene.” |
|
|
Dong et al. (2020b)38 |
Case report |
N:1 |
“It is reported a newborn with elevated IgM antibodies to SARS-CoV-2 born to a mother with coronavirus disease 2019 (COVID-19).” |
PCR |
a nasopharyngeal swab breast milk sample |
“Results from 5 RT-PCR tests on nasopharyngeal swabs taken from 2 hours to 16 days of age were negative.” “The mother’s breast milk had a negative RTPCR test result.” |
|
|
|
Perrone et al. (2020b)39 |
Case report |
N: 1 |
“It is reported the case of a mother who presented clinical symptoms of respiratory tract infection 10 days after the spontaneous delivery of a preterm newborn.” |
PCR |
nasopharyngeal swab breast milk sample |
“Since birth, the newborn was fed with both breastfeeding and expressed maternal milk.” “RT‐PCR assays on breast milk samples collected from affected women result negative for SARS‐CoV‐2.” |
“The adherence to hygiene measures by health workers and newborn parents, in addition to the paths implemented for the management of positive COVID‐19 patients, contributes to infection control and its spreading prevention.” |
|
|
Hinojosa-Velasco et al. (2020)40 |
Case report |
N:1 |
“This report describes a SARS-CoV-2 infection in a 21-year-old mother-daughter duo at the time of birth, focusing on the viral RNA detection in the stool of both and the human breast milk.” |
PCR |
nasopharyngeal and oropharyngeal swabs stool sample human milk sample |
“On the fourth day after delivery (May 25th), real-time RT-PCR analyses of the mother’s milk and stool samples were positive for SARS-CoV-2 RNA, a similar result was obtained for the infant’s stool sample.” |
|
|
|
Costa et al. (2020)41 |
Case report |
N:2 |
|
PCR |
amniotic fluid, cord blood, placental tissue, neonatal throat swab, and breast milk samples |
“In patient 1 we detected viral RNA in both placental tissue and cord blood samples and, importantly, in multiple breast-milk samples that were collected and tested after the first lactation.” “In patient 2 we did not detect viral RNA in any of the samples tested.” “All neonatal throat swab negative.” |
“Investigations of pregnant women with COVID-19 symptoms should necessarily include testing from various body sites or fluids.” |
|
|
Kuhrt et al. (2020)42 |
Case report |
N:2 (twins) |
“It is presented this case to highlight the following important issues: …placental pathology, the absence of vertical transmission in the context of preterm birth and placental abruption,...” |
PCR |
throat swab |
“The mother expressed breast milk. Both babies had a negative result for COVID-19 PCR test on postnatal days 3 and 5.” |
|
|
|
De Socio et al. (2020)43 |
Case report |
N:1 |
|
PCR |
Breast milk sample Nasopharyngeal swab |
“The newborn’s tested negative for SARS-CoV-2.” “The mother was assessed for SARS-CoV-2 RNA and resulted negative in the breast milk.” |
“Mother direct breastfeeding is advisable, under strict measures of infection control, i.e. a face mask should be worn due to the proximity between mother and child to reduce the risk of droplet transmission.” |
|
|
Pereira et al. (2020b)44 |
Case report |
N:22 |
“It was described the management of the mothers and babies during breastfeeding, the indication of breastfeeding depending on the severity of symptoms and finally breastfeeding in preterm neonates during NICU admission.” |
PCR |
nasopharyngeal swab |
“The experience shows that breastfeeding is safe with correct infection and control measures to decrease the risk of contagion by droplets and by contact with the respiratory secretions between mother and infant.” “Out of 22 mothers, 20 (90.9%) chose to breastfeed their babies during hospital admission.” “The newborn’s tested negative for SARS-CoV-2.” |
“When mother-baby child separation occurs, supplementing feeding with pasteurized donor human milk or infant formula may be effective until breastfeeding is resumed.” |
|
|
Kalafat et al. (2020)45 |
Case report |
N:1 |
|
PCR |
neonatal swabs, cord blood, breast milk sample and placental swab |
“The neonatal swabs, cord blood, breast milk sample and placental swab RT-PCR tests were negative for SARS-CoV-2.” |
|
|
|
Bastug et al. (2020)46 |
Case report |
N:1 |
“It is aimed to report the presence of SARS-CoV-2 in breast milk.” |
PCR |
breast milk sample |
“Breast milk obtained after first lactation was tested by real-time RT-PCR and was positive for SARS-CoV-2.” “SARS-CoV-2 PCR test of an NPS obtained from the infant was reported as negative.” |
“Although the benefits of breastfeeding may outweigh the risks, this decision should be made together by the parent and the doctor.” |
|
|
Buonsenso et al. (2020)47 |
Case report |
N:2 |
“This study aimed to evaluate postdischarge SARS-CoV-2 status of newborns to mothers with COVID-19 in pregnancy that, at birth, were negative to SARS-CoV-2.” |
PCR |
nasopharyngeal swabs |
“At birth and 3 days of life, newborns were negative to SARS-CoV-2. The mother breastfed him at home. At 2-week follow-up, one newborn tested positive although asymptomatic.” “Newborn 2, The father fed him at home with expressed milk. The newborn nasopharyngeal and rectal swabs were negative.” |
|
|
|
Zhang et al. (2020)48 |
Case report |
N:4 |
“It was identified all infected newborn babies in China by March 13 and described the clinical features, treatment, outcomes and intrauterine transmission potential.” |
PCR |
nasopharyngeal swabs or annals swabs |
“Four newborn babies tested positive in nucleic acid detection.” “Three newborn babies were separated from mothers right after being born and were not breastfed; one neonate had not been separated from mother and was breastfed for 16 days until symptom onset.” “Time between birth and diagnosis was limited, ranging between 30 hours and 5 days.” |
|
|
|
Salvatori et al. (2020)49 |
Case report |
N:2 |
“It is reported the first two maternal–infant dyads presenting to the emergency department because of a positive nasopharyngeal swab for SARS-CoV-2 both in the mother and in the child.” |
PCR |
breast milk samples nasopharyngeal swabs |
“It was analyzed expressed breast milk samples of both mothers, and SARS-CoV-2 was not detected by RT-PCR, as already described.” “Neonatal nasopharyngeal swab on admission positive.” |
“Whenever direct breastfeeding is not possible, the use of expressed mother’s milk should be considered and promoted to take advantage of its unquestionable benefits.” |
|
|
Tam P. C., et al. (2020)50 |
Case report |
N:1 |
“It was reported a case of detectable SARS-CoV-2 RNA in a woman with mild symptoms of COVID-19.” |
PCR |
oro-/nasopharyngeal swab human milk sample |
“Samples of the mother’s human milk none had detectable RNA.” “His nasopharyngeal swab was also positive for SARS-CoV-2 on RT-PCR testing.” “Upon confirmed COVID-19 infection in the infant, breastfeeding was resumed with no adverse effects.” |
|
|
|
Kirtsman et al. (2020)51 |
Case report |
N:3 |
|
PCR |
nasopharyngeal swab Placental tissue Breast milk sample |
“All 3 of the neonate’s nasopharyngeal swabs were positive for SARS-CoV-2 gene targets via RT-PCR testing.” “In the case, the mother’s nasopharyngeal swab, breast milk were positive for SARS-CoV-2 RNA.” |
“The woman attempted breastfeeding with appropriate precautions, including wearing a mask, practising appropriate hand and breast hygiene, and keeping the baby 2 m away from her between feedings.” |
|
|
Han M. S., et al. (2020)52 |
Case report |
N:1 |
“It was described the clinical manifestation of COVID-19 in a neonate and her mother, and further analyzed the viral load kinetics of SARS-CoV-2 in clinical specimens from different sources.” |
PCR |
nasopharynx, oropharynx, stool, saliva, plasma, and urine, breast milk sample |
“She was directly breastfed from birth.” “SARS-CoV-2 RNA was detected in the nasopharynx, oropharynx, stool, saliva, plasma, and urine.” “The virus was also not detected in her breast milk.” |
|
|
|
Chacón-Aguilar et al. (2020)53 |
Case report |
N:1 |
“It is presented one case of infection by coronavirus with an atypical course.” |
PCR |
nasopharyngeal swab |
“The polymerase chain reaction (PCR) test for detection of SARS-CoV-2 was positive.” “The infant was exclusively breastfed.” |
|
|
|
Zhu et al. (2020)54 |
Case report |
N:5 |
“It was reported detectable SARS-CoV-2 nucleic acid in human breastmilk from a puerperal woman with COVID-19.” |
PCR |
Breast milk sample |
“Four out of five (80%) patient‘s breastmilk samples were negative for SARS-CoV-2 RT-PCR.” “There were no infant outcomes reported.” |
|
|
|
Piersigilli et al. (2020)55 |
Case report |
N:1 |
“It is reported the case of an extremely preterm infant with COVID-19.” |
PCR |
nasopharyngeal swab breast milk sample |
“Maternal milk sample was negative.” “The SARS-CoV-2 rtPCR on her nasopharyngeal swab was positive.” |
“Contamination of breastmilk and other bodily fluids needs to be studied further, but the current policy is to allow the use of expressed breastmilk.” |
|
|
Bertinon et al. (2020)56 |
Case report |
N:14 |
“The aim was to look for the presence of SARS-CoV-2 RNA in the milk of a group of SARS-CoV-2 positive mothers from North-West Italy.” |
PCR |
nasopharyngeal swab breast milk sample |
“ In 13 cases the search for SARS-CoV-2 RNA in milk samples resulted negative and in one case it was positive. Thirteen of the 14 newborns were exclusively breastfed and closely monitored in the first month of life.” “Four newborns tested positive for SARS-CoV-2.” “Only one of the positive babies was breastfed.” |
“The recommended hygiene measures for the control of airborne exposure, for direct breastfeeding, must be carefully followed.” |
|
Table III: Child infection outcomes among articles not analysing breast milk for SARS-Cov-2 infection.
|
Neonates ≤28 days old |
Confirmed COVID-19 |
Negative COVID-19 |
Total |
Studies |
|
Breastfeeding |
10 |
324 |
334 |
Positive cases 14,20,47,48 |
|
Total |
10 |
324 |
334 |
|
|
*In the study of Pereira et al. (2020b),44 five babies received donor milk, and two babies received formula. |
||||
Table IV: Child infection outcomes among articles with breast milk samples analysed for SARS-Cov-2 infection.
|
Neonates ≤28 days old |
||||
|
|
Confirmed COVID-19 |
Negative COVID-19 |
Total |
Studies |
|
Breastfeeding |
13 |
60 |
73 |
Positive cases31,40, 41, 49**,51,52, 53, 55, 56 |
|
Neonates ≥28 days old |
||||
|
Breastfeedingc |
3 |
0 |
3 |
Positive cases29, 30, 50** |
|
Total |
16 |
60 |
76 |
|
|
*In the study of Bastug et al. (2020),46 breast milk was given to the baby only on the first day of birth. Later, when SARS-Cov-2 was positive in breast milk, formula was started. ** In the study, SARS-Cov-2 was not detected in breast milk in milk analysis. |
||||
The infection outcomes of breastfed neonates are summarised in Tables III and IV. Four hundred and ten breastfed neonates were identified, including 26 positive SARS-CoV-2 infection cases and 384 negative SARS-CoV-2 infection cases, based on the absence of viral RNA detection by RT-PCR tests. There were also three breastfed infants (infants older than 28 days) who were positive for SARS-CoV-2 infection.
Among the studies included in the systematic review, emphasised that mothers should take protective measures while breastfeeding.12,13,17,18,20,22,28,32,37,39,43,51,56 The use of a mask,12,22,32,37,43,51 hand hygiene,32,37,51 breast cleaning,51 taking hygienic measures,13,17,20,22,28,39,56 feeding by expressing breast milk,18 and keeping the infant away from the mother except for breastfeeding,51 were among the recommended protective measures. Furthermore, studies put forward that infants could additionally benefit from the direct acquisition of antibodies against SARS-CoV-2 through breast milk. It was recommended to provide mothers with their breast milk that could be given to infants during isolation and provide electronic breast pumps and breastfeeding support to allow the infant and/or mother to breastfeed directly after isolation.18 Wu et al. suggested that "mothers with COVID-19 should not breastfeed until after full recovery, when breast milk tests negative in terms of virus."26 Pereira et al. suggested that "when mother-baby separation occurs, supplementary feeding with pasteurised donor human milk or infant formula may be effective until breastfeeding is resumed."44
The infection outcomes of breastfed neonates are summarised in Table IV. In the studies analysing breast milk in the laboratory, while it was determined that 13 breastfed neonates (≤28 days old) and three infants aged 13.8 and 6 months outside the neonatal period had SARS-Cov-2 infection, SARS-Cov-2 infection was not detected in 60 neonates.
DISCUSSION
To the best of the authors’ knowledge, this is the first systematic review that examined suspected or confirmed COVID-19 infected mothers during the lactation period; and addressed whether it was safe to breastfeed/feed infants with breast milk.
In this study, a total of 1,229 articles were examined until November 15, 2020. Forty-six of which were assessed within the scope of the study. Among the 24 studies reviewed with breast milk samples taken, RT-PCR analysis reported that breast milk samples were positive for COVID-19 in seven studies and one sample had IgG (Table II). While the nasopharyngeal test result for COVID-19 was negative in a healthy neonate with SARS-CoV-2 in breast milk samples, SARS-CoV-2 was detected in breast milk samples.26 However, it is very difficult to determine the SARS-CoV-2 infection as the neonate receives breast milk. In another study, a neonate was determined to be positive for SARS-COV-2 based on viral RNA detected by RT-PCR, while being fed with maternal breast milk tested positive in terms of infection.40 In this specific case, the neonate was fed with synthetic milk formula instead of human milk. Therefore, it is unclear whether the neonate received the infection from its positive mother or contact with other patients with SARS-COV-2 infection.
It is an interesting fact that there were some infants with confirmed COVID-19 infection by RT- PCR tests who received SARS-CoV-2 negative breast milk, including two exclusively breastfed neonates,49 one 27-day breastfed neonate,52 and an infant aged 8 months.50 Therefore, it is thought that these infants may have been exposed to SARS-CoV-2 through close contact with infected relatives.
In line with the evidence obtained in articles not analysing breast milk samples, it cannot be confirmed whether there is an increase in the risk of SARS-CoV-2 transmission through breast milk among breastfed children. According to negative RT-PCR tests, most breastfed neonates did not have evidence of SARS-CoV-2 RNA (324 out of 334).
Only one study made conclusions and suggestions to keep the mother and infant apart.26 However, most of the available publications and the WHO, UNICEF, and WFO recommend that mothers with suspected or confirmed COVID-19 infection and isolated at home should continue feeding practices by taking necessary hygiene precautions during feeding.2,57
During the pandemic process, both pregnant women and breastfeeding mothers should be informed by a correct and reliable source on how to breastfeed/feed with breast milk, if they are in contact with the disease. The persons to whom they can refer in this regard are physicians, midwives, and nurses. However, in this rapidly developing process, healthcare professionals need reliable, evidence-based information to guide mothers in the right direction. Systematic reviews on this subject are important for meeting the needs of nurses and healthcare professionals who work very hard in this process. However, it should not be overlooked that the current information should be continuously updated with new and reliable studies in this process. The relevant systematic reviews are important to ensure the standardisation of nursing practices.
The limitation of this systematic review is that it is based on very recent studies on the subject. Despite the said limitation, the present comprehensive review consolidates important data to optimise outcomes better. This systematic review will be updated by including renewed publications. It is recommended to exclusively breastfeed infants for six months with complementary foods for up to 24 months and beyond. Among the studies, there were very few reports on infants outside the neonatal period. There is a need for a high level of evidence in a wide range of populations and different cultures, remaining current. A present systematic review allows us to incorporate relevant new evidence as it becomes available, which is crucial and rapidly increasing in the international health crisis.
CONCLUSION
In line with the limited data available, it was concluded that most of the infants breastfed had negative findings for SARS-Cov-2 infection in PCR, and breastfeeding mothers should initiate breastfeeding by taking protective measures such as wearing a mask and gloves and applying breast hygiene before breastfeeding and before contact with the infant.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
NC, OKS: Conceptualisation, writing original draft, writing review and editing, investigation, methodology, project administration, resources, visualisation, data curation.
SO, PT: Conceptualisation, writing original draft, writing review editing, and fund acquisition.
VC: Conceptualisation, writing original draft, writing review editing, and formal analysis.
REFERENCES
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 2020a; 323(13):1239-42. doi:10.1001/jama. 2020.2648.
- World health organization. (2020). Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020 (No. WHO/2019-nCoV/clinical/2020.4). Available from: https:// apps.who.int/iris/bitstream/handle/10665/331446/ WHO-2019-nCoV-clinical-2020.4-eng.pdf?sequence=1&isAllowed=y.
- https://covid19.who.int/
- Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. New Eng J Med 2020; 382(17):1663-5. doi:10.1056/NEJMc2005073.
- Huang C, Wang Y, Li X, Ren L, Zha J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395(10223):497-506. doi.org/10.1016/S0140-6736(20)30183-5.
- Chen L, Li Q, Zheng D, Jiang H, Wei , Zou L, et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. New Eng J Med 2020a; 382:25. doi:10.1056/ NEJMc2009226.
- Royal college of paediatrics and child health (2020) COVID-19 - guidance for neonatal settings https:// www.rcpch.ac.uk/sites/default/files/generated-pdf/document/COVID-19-guidance-for-neonatal-settings.pdf.
- Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020b; 395(10226):809-15. doi.org/10.1016/S0140-6736(20) 30360-3.
- Wang L, Shi Y, Xiao T, Fu J, Feng X, Mu D, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection. Ann Transl Med 2020a; 8(3). doi: 10.21037/atm.2020.02.20.
- Thomas BH, Ciliska D, Dobbins M, Micucci S. A process for systematically reviewing the literature: providing the research evidence for public health nursing interventions. Worldviews Evid Based Nurs 2004; 1(3):176-84. doi.org/10.1111/j.1524-475X.2004.04006.x.
- Ergin E, Akın B. The Turkish adaptation of a quality assessment tool for quantitative studies: Validity and reliability analyses. Turkiye Klinikleri Hemsirelik Bilimleri Dergisi 2018; 10(4):292-308. doi: 10.5336/nurses. 2018-61334.
- Ferrazzi E, Frigerio L, Savasi V, Vergani P, Prefumo F, Barresi S, et al. Vaginal delivery in SARS‐CoV‐2‐infected pregnant women in Northern Italy: A retrospective analysis. BJOG 2020; 127(9):1116-1121. doi.org/10.1111/ 1471-0528.16278.
- Gao X, Wang S, Zeng W, Chen S, Wu J, Lin X, et al. Clinical and immunologic features among COVID-19–affected mother–infant pairs: Antibodies to SARS-CoV-2 detected in breast milk. New Microbes New Infect 2020; 37:100752. doi.org/10.1016/j.nmni.2020.100752.
- Savasi VM, Parisi F, Patane L, Ferrazzi E, Frigerio L, Pellegrino A, et al . Clinical findings and disease severity in hospitalised pregnant women with coronavirus disease 2019 (COVID-19). Obstet Gynecol 2020; 136(2):252-8. doi.org/ 10.1097/AOG.0000000000003979.
- Sahin, D, Tanacan A, Erol SA, Anuk AT, Eyi EG, Ozgu‐Erdinc AS, et al. Pandemic center’s experience of managing pregnant women with COVID‐19 infection in Turkey: A prospective cohort study. Int J Gynecol Obstet 2020; 151(1):74-82. doi.org/10.1002/ijgo.13318.
- Hirshberg JS, Stout MJ, Raghuraman N. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: Two weeks of confirmed presentations to an affiliated pair of New York City Hospitals. Am J Obstet Gynecol Mfm 2020; 2(3): doi.org/10.1016/j.ajogmf.2020. 100162.
- Salvatore CM, Han JY, Acker KP, Tiwari P, Jin J, Brandler M, et al. Neonatal management and outcomes during the COVID-19 pandemic: An observation cohort study. Lancet Child Adolesc Health 2020; 4(10):721-7. doi.org/10. 1016/S2352-4642(20)30235-2.
- Griffin I, Benarba F, Peters C, Oyelese Y, Murphy T, Contreras D, et al. The impact of COVID-19 infection on labor and delivery, newborn nursery, and neonatal intensive care unit: prospective observational data from a single hospital system. Am J Perinatol 2020; 37(10):1022. doi:10. 1055/s-0040-1713416.
- Cojocaru L, Crimmins S, Sundararajan S, Goetzinger K, Elsamadicy E, Lankford A, et al. An initiative to evaluate the safety of maternal bonding in patients with SARS-CoV-2 infection. J Matern Fetal Neonatal Med 2020; 1-7. doi.org/10.1080/14767058.2020.1828335.
- Anand P, Yadav A, Debata P, Bachani S, Gupta N, Gera R. Clinical profile, viral load, management and outcome of neonates born to COVID 19 positive mothers: A tertiary care centre experience from India. Eur J Pediatr 2020; 1-13. doi.org/10.1007/s00431-020-03800-7.
- Pereira A, Cruz‐Melguizo S, Adrien M, Fuentes L, Marin E, Perez‐Medina T. Clinical course of Coronavirus disease‐2019 (COVID‐19) in pregnancy. Acta Obstetricia et Gynecologica Scandinavica 2020a; 99(7):839-47. doi.org/10.1111/aogs. 13921.
- Marin Gabriel MA, Cuadrado I, Álvarez Fernández B, González Carrasco E, Alonso Díaz C, Llana Martín I. Neo‐COVID‐19 research group. Multicentre spanish study found no incidences of viral transmission in infants born to mothers with COVID‐19. Acta Paediatr 2020a; 109(11): 2302-2308. doi.org/10.1111/apa.15474.
- Farghaly MA, Kupferman F, Castillo F, Kim RM. Characteristics of newborns born to SARS-CoV-2-positive mothers: A retrospective cohort study. Am J Perinatol 2020; 37(13):1310-6 doi.org/10.1055/s-0040-1715862.
- Patil UP, Maru S, Krishnan P, Carroll-Bennett R, Sanchez J, Noble L, et al. Newborns of COVID-19 mothers: Short-term outcomes of colocating and breastfeeding from the pandemic’s epicenter. J Perinatol 2020; 40(10):1455-8. doi.org/10.1038/s41372-020-0765-3.
- Dumitriu D, Emeruwa UN, Hanft E, Liao GV, Ludwig E, Walzer L, et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical centre in New York City. JAMA Pediatr 2020; 175(2):157-67 doi.org/10.1001/jamapediatrics. 2020.4298.
- Wu Y, Liu C, Dong L, Zhang C, Chen Y, Liu J, et al. Coronavirus disease 2019 among pregnant Chinese women: Case series data on the safety of vaginal birth and breastfeeding. BJOG: An Int J Obstet Gynaecol 2020b; 127(9):1109-15. doi.org/10.1111/1471-0528.16276.
- Li Y, Zhao R, Zheng S, Chen X, Wang J, Sheng X, et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis 2020; 26(6):1335-6. doi: 10.3201/eid2606.200287.
- Lowe B, Bopp B. COVID‐19 vaginal delivery–a case report. Aust N Z J Obstet Gynaecol 2020; 60(3):465-6. doi.org/10.1111/ajo.13173.
- Yu Y, Li Y, Hu Y, Li B, Xu J. Breastfed 13 month-old infant of a mother with COVID-19 pneumonia: A case report. Int Breastfeeding J 2020; 15(1):1-6. doi.org/10.1186/ s13006-020-00305-9.
- Kam KQ, Yung CF, Cui L, Tzer Pin Lin R, Mak TM, Maiwald M, et al. A well infant with coronavirus disease 2019 with high viral load. Clin Infect Dis 2020; 71(15):847-9. doi.org/10. 1093/cid/ciaa201.
- Wang S, Guo L, Chen L, Liu W, Cao Y, Zhang J, et al. A case report of neonatal 2019 coronavirus disease in China. Clin Infect Dis 2020b; 71(15):853-7. doi.org/10.1093/ cid/ciaa225.
- Lebrao CW, Cruz MN, Silva MHD, Dutra LV, Cristiani C, Affonso Fonseca FL, et al. Early Identification of IgA Anti-SARSCoV-2 in milk of mother with COVID-19 Infection. J Hum Lact 2020; 36(4):609-13. doi.org/10.1177/0890 334420960433.
- Perrone S, Giordano M, Meoli A, Deolmi M, Marinelli F, Messina G, et al. Lack of viral transmission to preterm newborn from a COVID‐19 positive breastfeeding mother at 11 days postpartum. J Med Virol 2020a; 92(11):2346-7. doi.org/10.1002/jmv.26037.
- Dong L, Tian J, He S, Zhu C, Wang J, Liu C, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 2020a; 323(18):1846-8. doi.org/10.1001/jama.2020.4621.
- Al-kuraishy HM, Al-Maiahy TJ, Al-Gareeb AI, Musa RA, Ali ZH. COVID-19 pneumonia in an Iraqi pregnant woman with preterm delivery. Asian Pacific J Reproduction; 2020; 9(3): 156. doi.org/10.4103/2305-0500.282984.
- Marin Gabriel MÁ, Malalana Martínez AM, Marín Martínez ME, Anel Pedroche J. Negative transmission of SARS-CoV-2 to hand-expressed colostrum from SARS-CoV-2–positive mothers. Breastfeed Med 2020b; 15(8):492-4. doi.org/10. 1089/bfm.2020.0183.
- Chhabra A, Rao TN, Kumar M, Singh Y, Subramaniam R. Anaesthetic management of a COVID-19 parturient for caesarean section-case report and lessons learnt. Indian J Anaesth 2020; 64(14):141. doi.org/10.4103/ija.IJA_509_20.
- Dong Y, Chi X, Hai H, Sun L, Zhang M, Xie WF, et al. Antibodies in the breast milk of a maternal woman with COVID-19. Emerg Microbes Infect 2020b; 9(1):1467-9. doi.org/10.1080/22221751.2020.1780952.
- Perrone S, Deolmi M, Giordano M, D’Alvano T, Gambini L, Corradi M, et al. Report of a series of healthy term newborns from convalescent mothers with COVID-19. Acta Bio Medica Atenei Parmensis 2020b; 91(2):251. doi: 10.23750/abm.v91i2.9743.
- Hinojosa-Velasco A, de Oca PVBM, García-Sosa LE, Mendoza-Durán, JG, Pérez-Méndez MJ, Dávila-González E, et al. A case report of newborn infant with severe COVID-19 in Mexico: Detection of SARS-CoV-2 in human breast milk and stool. Intern J Infec Dis 2020; 100:21-4. doi.org/10. 1016/j.ijid.2020.08.055.
- Costa S, Posterar B, Marchett S, Tamburrini E, Carducci B, Lanzone A, et al. Excretion of SARS-CoV-2 in human breast milk. Clin Microbiol Infect 2020; 26:1430-2. https:// doi.org/10.1016/j.cmi.2020.05.027.
- Kuhrt K, McMicking J, Nanda S, Nelson-Piercy C, Shennan A. Placental abruption in a twin pregnancy at 32 weeks’ gestation complicated by coronavirus disease 2019 without vertical transmission to the babies. Am J Obstet Gynecol MFM 2020; 2(3):100135. doi.org/10.1016/j.ajogmf. 2020.100135.
- De Socio GV, Malincarne L, Arena S, Troiani S, Benedetti S, Camilloni B, et al. Delivery in asymptomatic Italian woman with SARS-CoV-2 infection. Mediterr J Hematol Infect Dis 2020; 12(1):e2020033. dx.doi.org/10.4084/MJHID.2020. 033.
- Pereira A, Cruz-Melguizo S, Adrien M, Fuentes L, Marin E, Forti A, et al. Breastfeeding mothers with COVID-19 infection: A case series. Int Breastfeeding J 2020b; 15(1):1-8. doi.org/10.1186/s13006-020-00314-8.
- Kalafat E, Yaprak E, Cinar G, Varli B, Ozisik S, Uzun C, et al. Lung ultrasound and computed tomographic findings in pregnant woman with COVID‐19. Ultrasound Obstetrics Gynecol 2020; 55(6):835-7. doi.org/10.1002/uog.22034.
- Bastug A, Hanifehnezhad A, Tayman C, Ozkul A, Ozbay O, Kazancioglu S, et al. Virolactia in an asymptomatic mother with COVID-19. Breastfeed Med 2020; 15(8):488-91. doi.org/10.1089/bfm.2020.0161.
- Buonsenso D, Costa S, Sanguinetti M, Cattani P, Posteraro B, Marchetti S, et al. Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am J Perinatol 2020; 37(8):869. doi.org/10.1055/ s-0040- 1710541.
- Zhang ZJ, Yu XJ, Fu T, Liu Y, Jiang Y, Yang BX, et al. Novel coronavirus infection in newborn babies under 28 days in China. Eur Respiratory J 2020; 55:2000697 doi.org/10.1183/ 13993003.00697-2020.
- Salvatori G, De Rose DU, Concato C, Alario D, Olivini N, Dotta A, et al. Managing COVID-19-positive maternal–ınfant dyads: An Italian experience. Breastfeed Med 2020; 15(5):347-8. doi.org/10.1089/bfm.2020.0095.
- Tam PC, Ly KM, Kernich ML, Spurrier N, Lawrence D, Gordon D, et al. Detectable severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human breast milk of a mildly symptomatic patient with coronavirus disease 2019 (COVID-19). Clin Infect Dis 2020; 72(1):128-30. doi.org/10. 1093/cid/ciaa673.
- Kirtsman M, Diambomba Y, Poutanen SM, Malinowski AK, Vlachodimitropoulou E, Parks WT, et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ 2020; 192(24) E647-50. doi.org/10.1503/cmaj.200821.
- Han MS, Seong MW, Heo EY, Park JH, Kim N, Shin S, et al. Sequential analysis of viral load in a neonate and her mother infected with SARS-CoV-2. Clin Infect Dis 2020; 71(6):2236-9. doi.org/10.1093/cid/ciaa447.
- Chacón-Aguilar R, Osorio-Cámara JM, Sanjurjo-Jimenez I, González-González C, López-Carnero J, Pérez-Moneo B. COVID-19: Fever syndrome and neurological symptoms in a neonate. An Pediatr 2020; 92(6):372-4. doi.org/10. 1016/j.anpede.2020.04.001.
- Zhu C, Liu W, Su H, Li S, Shereen MA, Xia Y. Breastfeeding risk from detectable severe acute respiratory syndrome Coronavirus 2 in Breastmilk. J Infect 2020; 81(3):452-82 doi.org/10.1016/j.jinf.2020.06.001.
- Piersigilli F, Carkeek K, Hocq C, van Grambezen B, Hubinont C, Chatzis O, et al. COVID-19 in a 26-week preterm neonate. Lancet Child Adolesc Health 2020; 4(6):476-8. doi.org/ 10.1016/S2352-4642(20)30140-1.
- Moro B, De Renzi G, Viberti Cavallo R, Coscia A, Soldi A. Detection of SARS-CoV-2 in Milk From COVID-19 positive mothers and follow-up of their ınfants. Front Pediatr 2020; 8:676. doi.org/10.3389/fped.2020.597699.
- United Nations International children's emergency fund. Breastfeeding safely during the COVID-19 pandemic 08 September 2020. https://www.unicef.org/coronavirus/ breastfeeding-safely-during-covid-19-pandemic.