Atypical Presentation of a Primary Thymic Adenocarcinoma
By Hasan Ersoz1, Ebru Cakir2, Utku Oflazoglu3, Berna Dernek4Affiliations
doi: 10.29271/jcpsp.2022.08.S141ABSTRACT
Primary thymic adenocarcinomas are extremely rare and aggressive tumours. In this report, clinical and histopathological findings of this rare entity as well as its treatment approaches are presented. A 55-year man was operated for anterior mediastinal mass. Histopathological examination revealed primary thymic adenocarcinoma. No other tumour focus was found in clinical, radiological, and endoscopic examinations. Due to the capsular invasion, adjuvant mediastinal radiotherapy was applied. In the process of monitoring, brain metastases developed. Stereotactic radiosurgery and chemotherapy were applied. All the lesions are under control at 28 months postoperatively.
Complete surgical resection of the tumour is the most important factor. Adjuvant radiotherapy and/or chemotherapy as well as local therapies for metastases (surgical therapy or stereotactic radiosurgery) are seen as approaches to improve survival.
Key Words: Adenocarcinoma, Metastasis, Stereotactic radiosurgery, Thymus.
INTRODUCTION
The incidence of thymic carcinomas is about 1-3 out of ten-million.1 Primary thymic adenocarcinomas are an extremely rare subtype that accounts for 1.6% of all thymic carcinomas.1 The information about these tumors is extremely limited. Primary thymic adenocarcinomas were first reported in 1989, but were not considered a valid histological subtype until 1997. In this report, histopathological and clinical features of the case of primary thymic adenocarcinoma in a 55-year male patient is discussed along with its management.
CASE REPORT
A 55-year man presented with a complaint of difficulty in swallowing and right shoulder pain. Thoracic computed tomography (CT) showed a mass (37×44 mm) in the anterior mediastinum (Figure 1a).
Positron emission tomography (PET) showed increased F-18 fluorodeoxyglucose (FDG) uptake in the mass without any other pathological involvement. Cranial magnetic resonance imaging (MRI) was normal. The mass was excised with median sternotomy. Due to the right pulmonary adherence of the mass, wedge resection with a two cm safety margin was performed also. Thus, the adherent part of the lung and the mediastinal mass were excised in one piece (Figure 1b).
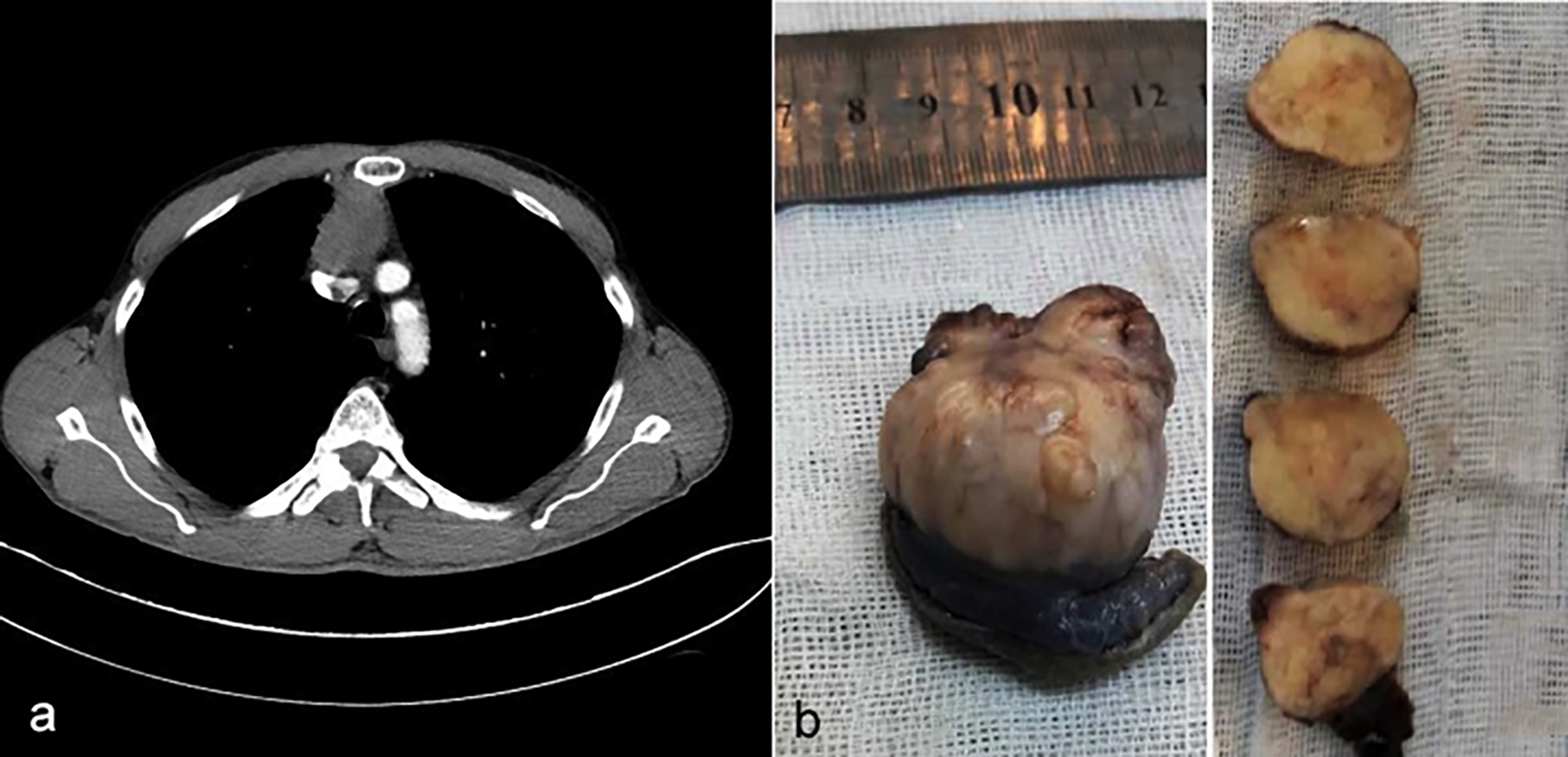 Figure 1: (a) The tumor on thoracic computed tomography. (b) Nodular mediastinal mass with solid gray tan surface partially surrounded by the lung tissue.
Figure 1: (a) The tumor on thoracic computed tomography. (b) Nodular mediastinal mass with solid gray tan surface partially surrounded by the lung tissue.
Microscopical examination revealed a moderately differentiated non-mucinous adenocarcinoma consisting of tubular and tubulopapillary structures lined by columnar cells in the desmoplastic stroma containing inflammatory cells (Figure 2a). The tumor was separated from the lung parenchyma by thickened pleura showing fibrosis (Figure 2b). Microscopic thymoma focus and millimeter-sized thymic cyst were noted in the residual thymic tissue around the tumor. The tumor cells were diffusely positive with CK7 and CEA (membranous), and focal positive with CD5 and CDX2. TTF1, Napsin A, calretinin, p63, CD117, and CK20 were negative. Microscopically, there was no tumor at the surgical margins. When histopathological findings were evaluated together with the immunohistochemical panel, the tumor was reported as thymic adenocarcinoma, NOS; however, it was suggested that the metastasis should be excluded clinically.
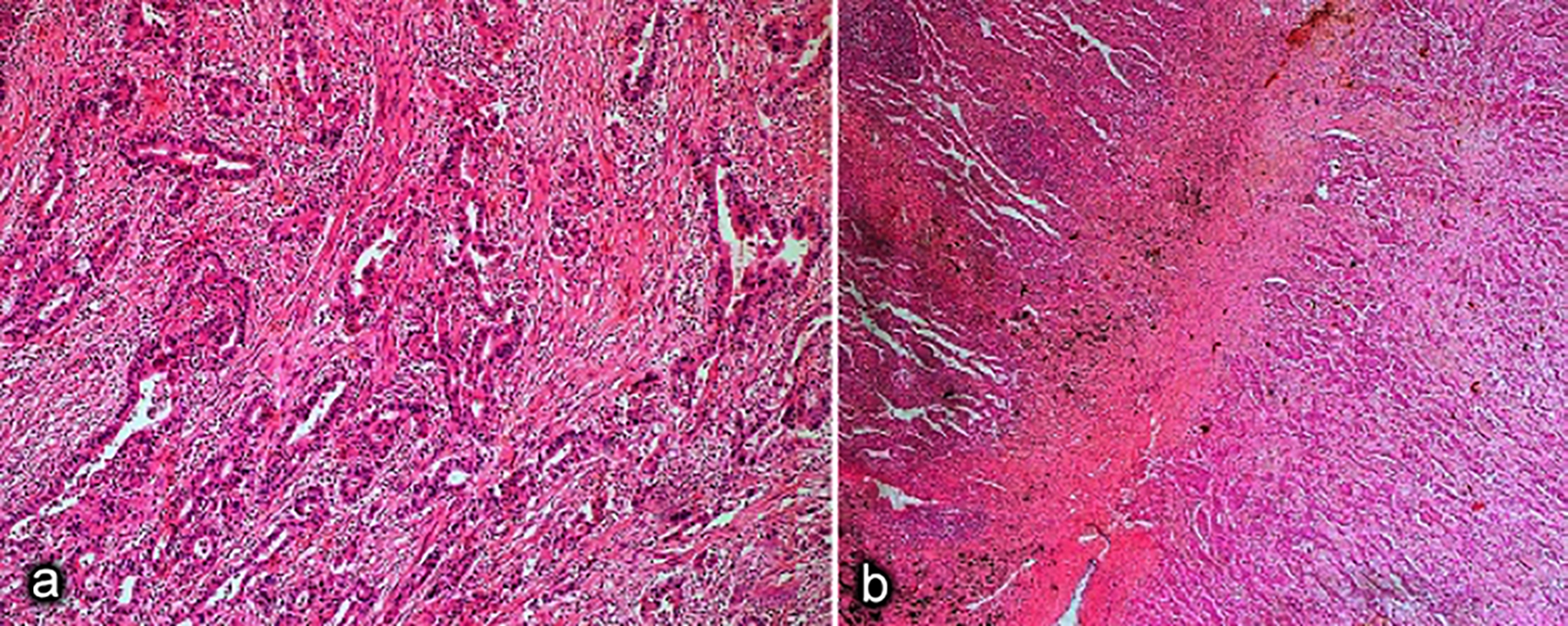 Figure 2: (a) Tumor composed of tubules and tubulopapillary structures lined by columnar cells in a lymphoplasmacyte and neutrophil-rich stroma (H&E, ×200). (b) Tumor was well demarcated from the lung parenchyma by a fibrotic stroma (H&E, ×40).
Figure 2: (a) Tumor composed of tubules and tubulopapillary structures lined by columnar cells in a lymphoplasmacyte and neutrophil-rich stroma (H&E, ×200). (b) Tumor was well demarcated from the lung parenchyma by a fibrotic stroma (H&E, ×40).
Endoscopy and colonoscopy were performed to exclude possible metastatic adenocarcinomas. There was no primary focus in these regions and the case was considered as a primary thymic adenocarcinoma. Since the tumor had capsular invasion, 54 Gray (Gy) Intensity Modulated Radiotherapy (IMRT) was applied to the mediastinal region with Trilogy Device.
At the sixth postoperative month, cranial MRI showed two newly developed metastatic lesions. Since the lesions were surgically inoperable, 22 Gy stereotactic radiosurgery (SRS) was applied with Cyberknife Device. Subsequently, carboplatin (5 AUC) and paclitaxel (175 mg/m2) were administered. After four cycles of chemotherapy (at 12th month postoperatively), the cranial MRI showed complete resolution of the two previous lesions but also the development of two new metastatic lesions. Eighteen Gy SRS was applied to these lesions. The chemotherapy agents were changed [doxorubicin (50 mg/m2), cyclophosphamide (500 mg/m2) and cisplatin (50 mg/m2)].
In the cranial MRI at 17th month postoperatively, all the metastatic lesions had regressed. The patient is being followed up without any disease at 28th month postoperatively.
DISCUSSION
Primary thymic adenocarcinomas were first reported in 1989, but were not considered a valid histological subtype until 1997. These tumors are most commonly seen in the 4-5th decades. Symptoms commonly observed are dyspnea, chest pain, and cough. 1 Our case was also a 5th decade patient but had atypical symptoms.
The presence of a microscopic thymic cyst and thymoma concurrently is supportive of primary thymic adenocarcinoma.1 There was no tumour in the lung parenchyma and no germ cell component was identified in the tumor. In addition, immunohistochemical findings supported the thymic origin. The clinical exclusion was recommended for a gastrointestinal primary tumour because of focal CDX2 positivity.
Surgery is the mainstay of treatment in primary thymic adenocarcinomas.2,3 Adjuvant radiotherapy is effective to increase local control in the high-risk group of patients.4 The presence of capsular invasion was an indication of radiotherapy. However, since no distant metastasis was detected at that time, systemic chemotherapy was not considered.
We believe that this local treatment is successful because there has been no recurrence in the mediastinal region. However, brain metastasis developed afterward. The following question comes to mind about this situation: Would it have been better if adjuvant chemotherapy had been administered?
It has been reported that brain metastases develop in thymic carcinomas and adenocarcinomas at a frequency of 17.6 and 25%, respectively.1 Surgical excision is the best treatment. SRS is considered an alternative option for inoperable patients with brain metastasis.5 Therefore, SRS was attempted for the first two brain metastases and supplemented with carboplatin-paclitaxel chemotherapy. Although new lesions occurred subsequently, the first two lesions regressed with this treatment. This situation brought to mind two considerations. Firstly, the platinum-based-chemotherapy was unsuccessful, and secondly, SRS was highly effective in controlling brain metastases. For this reason, second-line chemotherapy was initiated afterward.
The reported results of chemotherapy for thymic adenocarcinomas are not successful. In a report of nine patients, only three cases survived for more than five years.2 In another study, the results are almost similar.3 In both studies, the importance of an R0 surgery was emphasised.
In conclusion, an accurate diagnosis, R0 excision, and if necessary, adjuvant mediastinal radiotherapy are appropriate management approaches for these rare primary thymic neoplasms. It should be kept in mind that brain metastases may develop in these tumors. Therefore, cranial imaging should be included as part of systemic screening in these patients. SRS may be an effective treatment approach for brain metastases as an alternative to surgery.
PATIENT’S CONSENT:
Written informed consent for patient information and images to be published was provided by the patient.
COMPETING INTEREST:
The authors declared no competıng interest.
AUTHORS’ CONTRIBUTION:
HE: Concept, primary surgeon, literature search, manuscript writing, editing, and final approval.
EC: Concept, histopathological diagnosis, literature search, manuscript writing, and editing.
UO: Primary medical oncology, literature search, manuscript writing, and editing.
BD: Primary radiation oncology, literature search, manuscript writing, and editing.
REFERENCES
- Kalhor N, Moran CA. Primary thymic adenocarcinomas: A clinicopathological and immunohistochemical study of 16 cases with emphasis on the morphological spectrum of differentiation. Hum Pathol 2018; 74:73-82. doi: 10.1016/j. humpath.2018.01.011.
- Kinoshita F, Shoji F, Takada K, Toyokawa G, Okamoto T, Yano T, et al. Mucinous adenocarcinoma of the thymus: report of a case. Gen Thorac Cardiovasc Surg 2018; 66(2): 111-5. doi: 10.1007/s11748-017-0781-1.
- Maeda D, Ota S, Ikeda S, Kawano R, Hata E, Nakajima J, et al. Mucinous adenocarcinoma of the thymus: A distinct variant of thymic carcinoma. Lung Cancer 2009; 64(1): 22-7. doi: 10.1016/j.lungcan.2008.06.019.
- Ettinger DS, Riely GJ, Akerley W, Borghaei H, Chang AC, Cheney RT, et al. Thymomas and thymic carcinomas: Clinical practice guidelines in oncology. J Natl Compr Canc Netw 2013; 11(5):562-76. doi: 10.6004/jnccn.2013.0072.
- Lo SS, Chang EL, Sahgal A. Radiosurgery for resected brain metastases-a new standard of care? Lancet Oncol 2017; 18(8):985-7. doi: 10.1016/S1470-2045(17)30448-5.