Association between Serum Carcino-embryonic Antigen Levels and Micropapillary Bladder Cancer Metastasis in an Elderly Male
By Caner Ediz1, Neslihan Kaya Terzi2, Serkan Akan1, Omer Yilmaz1Affiliations
doi: 10.29271/jcpsp.2022.08.S186ABSTRACT
We, herein, present a case of a micropapillary variant of bladder cancer metastasizing to lymph nodes in an 87-year male with elevated serum carcinoembryonic antigen (CEA) levels (2637.8 ng/mL). The patient was evaluated for dyspeptic symptoms and elevated CEA levels. Colonoscopy and upper gastrointestinal endoscopy were normal. Contrast-enhanced computed tomography revealed a bladder tumour. Transurethral resection of bladder tumour (TUR-BT) was performed, and histologically, the tumour was reported as urothelial carcinoma (UC), high grade, and pT1. Intravesical Bacillus Calmette-Guérin (BCG) was started three weeks after TUR-BT and continued for two years. F-18 FDG PET/CT scans were performed every six months during the follow-up due to persistently elevated CEA levels. During follow-up, there was no recurrence of UC in the bladder. Two years later, he was admitted again with lymph node swelling in the left inguinal area. A tru-cut biopsy was performed, which showed UC with a micropapillary component. Gemcitabine monotherapy was given, which resulted in partial response, and a significant decline in serum CEA levels (490.17 ng/mL).
Key Words: Carcinoembryonic antigen, Urothelial carcinoma, Bladder cancer, Micropapillary variant, Gemcitabine monotherapy.
INTRODUCTION
The micropapillary variant of urothelial carcinoma (UC) is a rarely diagnosed, aggressive tumour and usually presents at an advanced stage. This variant may also be diagnosed first as a metastases as well as in the bladder. This tumour and/or its metastases have been reported that cause elevated serum carcinoembryonic antigen (CEA) levels in very few cases in the literature. Intravesical therapy, external beam radiotherapy, and chemotherapy are used in the management of micropapillary UC.1 In this case report, we present a case of the partial response of the elevated serum CEA levels to gemcitabine monotherapy in a case with lymph node metastasis involving the micropapillary variant of UC.
CASE REPORT
An 87-year male presented with dyspeptic complaints in July 2017. Serum tumour markers were evaluated for suspected gastrointestinal tumour. Serum CEA level was found to be 65.09 (Normal: 0-5) ng/mL. Other laboratory tests were normal. Colonoscopy and upper gastrointestinal endoscopy were performed to evaluate the digestive tract. Both of them were normal and samples from suspicious areas in the digestive tract were reported as inflammatory lesions. A 12×16 mm bladder mass in contrast-enhanced computed tomography, which was performed to evaluate the rise of CEA, was incidentally detected. On cystoscopic evaluation, wide-based, and calcified tumour mass was found, and transurethral resection of bladder tumour (TUR-BT) was performed.
The histology showed moderately differentiated UC with papillary configuration. Tumor cells were proliferating in large and small irregular solid nests, with marked pleomorphism, marked variation in size and shape, with many mitotic figures (Figure 1A). A micropapillary carcinoma (MPC) component without fibrovascular cores was observed in a small area of the mass (Figure 1B). Immunohistochemically, CK7 (Figure 1C), GATA-3, Uroplakin II, and p63 were positive in all biopsies. The staining pattern of the micropapillary component was nearly identical to that of the papillary UC component, but the positive reaction for polyclonal and monoclonal CEA (p-CEA and m-CEA) was less intense in the micropapillary component than in the papillary UC component (Figure 1D).
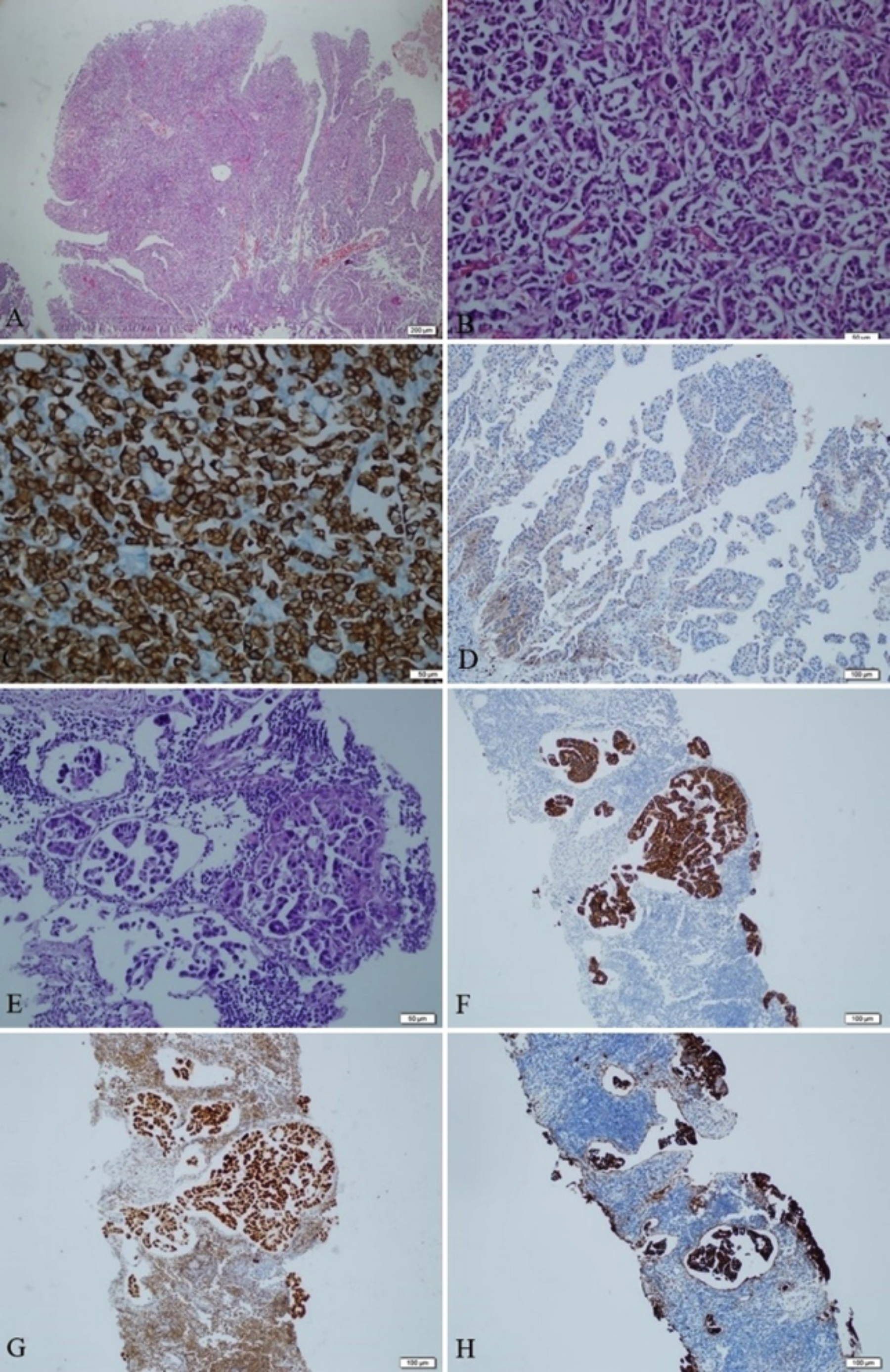 Figures 1: Histopathological and immunohistochemical features of primary and metastatic tumour. (A) Cancer cells proliferating in irregular nests and papillary pattern, with marked invasion. (Hematoxylin and eosin [H&E], ×200). (B) In this area of the mass, a micropapillary component without fibrovascular cores is observed (H&E, ×200). (C) Cancer cells were positive on anti-CK7 antibody staining. (Anti-CK7 antibody, ×400). (D) The staining pattern of the micropapillary component was weak and negative for CEA. (E) Microscopic findings of the trucut biopsy of the lymph node. Biopsy consisted of micropapillary component only (H&E, ×200). (F&G) Cancer cells were positive on anti-CK7 and GATA-3 antibody staining. (Anti-CK7 and GATA-3 antibody, ×100). H Metastatic tumour consisting of micropapillary component showed strong staining with CEA.
Figures 1: Histopathological and immunohistochemical features of primary and metastatic tumour. (A) Cancer cells proliferating in irregular nests and papillary pattern, with marked invasion. (Hematoxylin and eosin [H&E], ×200). (B) In this area of the mass, a micropapillary component without fibrovascular cores is observed (H&E, ×200). (C) Cancer cells were positive on anti-CK7 antibody staining. (Anti-CK7 antibody, ×400). (D) The staining pattern of the micropapillary component was weak and negative for CEA. (E) Microscopic findings of the trucut biopsy of the lymph node. Biopsy consisted of micropapillary component only (H&E, ×200). (F&G) Cancer cells were positive on anti-CK7 and GATA-3 antibody staining. (Anti-CK7 and GATA-3 antibody, ×100). H Metastatic tumour consisting of micropapillary component showed strong staining with CEA.
Intravesical Bacillus Calmette-Guérin (BCG) was started three weeks after TUR-BT and continued for two years. In this period, the control cystoscopy was repeated every three months. Three months after initial TUR-BT, F-18 FDG PET/CT was done due to elevated serum CEA levels (2637.8 ng/mL) and did not show increased uptake in any area. F-18 FDG PET/CT and serum CEA levels were reassessed six months later. Hypermetabolic lymph nodes up to 14×13 mm in the right paratracheal, prevascular, bilateral hilar, paraaortic, interaortocaval and retrocaval regions were detected. Approximately 19×12 mm hypermetabolic lymph node was also reported in the left inguinal region. Therefore, CEA levels and F-18 FDG PET/CT were performed every six months during the follow-up. Additional treatment was not offered because of the patient's advanced age, and watchful waiting was advised. No recurrence of tumour in the bladder occurred in the follow-up. However, serum CEA levels (6095.46 and 8624.96 ng/mL, respectively) and maximum standardised uptake values (SUVmax) in F-18 FDG PET/CT (5.8 and 6.6, respectively) steadily increased. A core needle biopsy was performed of the hypermetabolic lymph node in the left inguinal region. The MPC component was predominant in the metastatic lesions (Figure 1E). Positivity was observed in the same manner as antibodies applied to the previous biopsy. No differences were found in immunostaining for CK7 and GATA-3, and staining with these antibodies was positive for all cancer cells, including those of the MPC component (Figures 1F&1G). Unlike the resection material, the micropapillary component was immunohistochemically stained with CEA (Figure 1H).
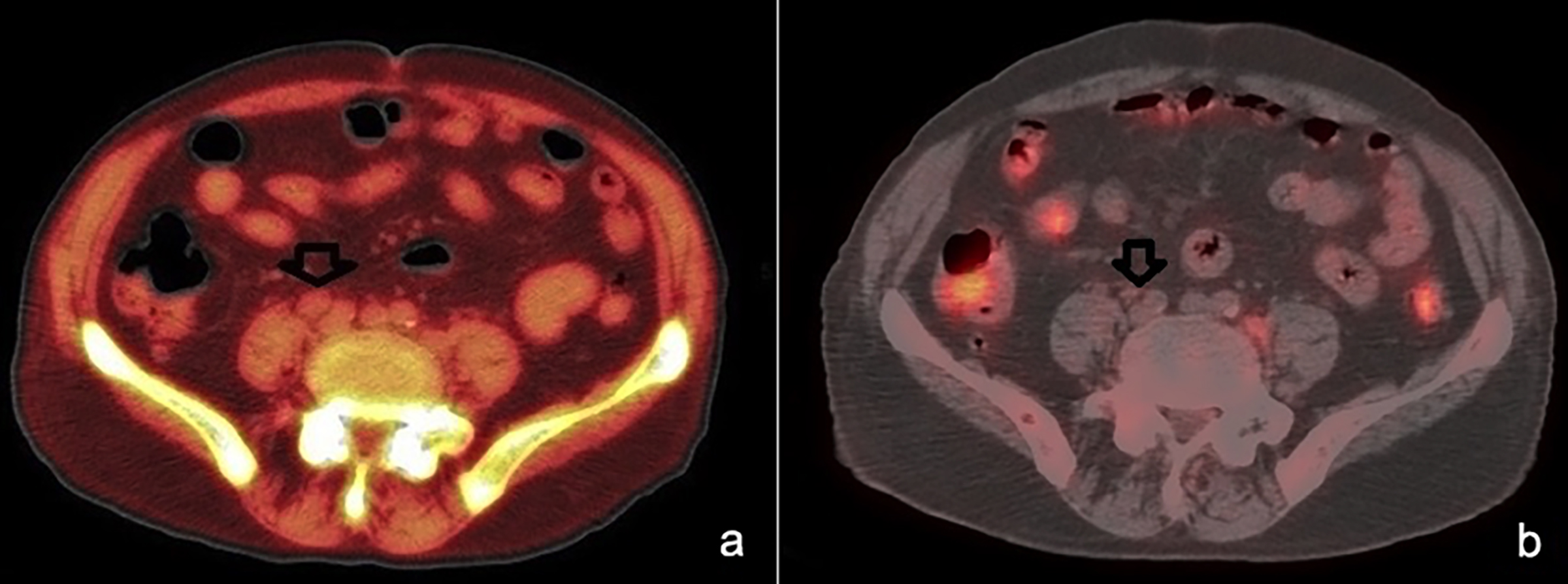 Figure 2: Status of lymphadenopathies in the intra abdominal area before (a) and after chemotherapy (b).
Figure 2: Status of lymphadenopathies in the intra abdominal area before (a) and after chemotherapy (b).
Chemotherapy (CT) treatment was started due to steadily increasing serum CEA levels, high SUVmax values, and hypermetabolic lymph nodes that contained MPC. Gemcitabine monotherapy infusion at a dose of 1000 mg/m2 over 30 min (the standard regimen) on days 1 and 8 of every 3-week cycle was preferred because of the patient's advanced age. After six cycles of CT, serum CEA levels decreased to 490.17 ng/mL, SUVmax in F-18 FDG PET/CT was 3.5, and lesions partially regressed on computed tomography (Figure 2). Also, after chemotherapy, dyspeptic complaints partially regressed. Radiotherapy was not considered because of extensive lesions and gastrointestinal complaints of the patient.
DISCUSSION
The defined CEA reference range for elderly (61-89 years) patients is 0-5.98 ng/mL.2 Increased levels of CEA may be found in colorectal or colon cancer, medullary thyroid carcinoma, breast cancer, cancer of the gastrointestinal tract, liver cancer, lung cancer, ovarian cancer, pancreatic cancer, and prostate cancer. The association of serum CEA levels with bladder cancer has been reported very rarely. Association between high serum levels of CEA and T1 high-grade UC was reported in 2008 by Koizumi et al. and in some cases, CEA was found positive in immunohistochemical analysis of tissue samples in MPC of the bladder UC.3-5
In this case, there was no recurrence in the bladder after BCG treatment. Although the MPC component was not reported in the bladder mass, it was present in a small area in the bladder mass (less than 1%). The serum CEA level did not significantly regress after the TUR-BT, and it steadily increased in the follow-up due to the MPC component of occult metastasis.
Micropapillary bladder cancer (MPBC), even when detected in less than 10% of UC within the TUR-BT sample, is associated with poor outcomes.4 Therefore, some authors argue for proactive immediate radical cystectomy in patients with T1 high-grade UC with MPC component6,7 and others assert to use BCG therapy.1,8 We preferred BCG treatment due to the advanced age of the patient. Although the bladder was normal in follow-up, occult metastases progressed to multiple lymph nodes. The metastatic lesions were not detected on F-18 FDG PET/CT until CEA levels reached approximately 6000 ng/mL.
Chemotherapy (cisplatin, gemcitabine, carboplatin, metho-trexate or teicoplanin etc. ) is a primary method of treatment of the metastases in UC. The overall response of gemcitabine monotherapy in metastatic bladder cancer ranges from 11% to 25% in some studies.9,10 The presence of metastasis in a limited area may be suitable for the radiotherapy (RT) option. Koizumi et al. reported a complete response of MPC component in lymph nodes by radiotherapy treatment.3 In our case, RT was not preferred due to multiple metastatic lesions. However, gemcitabine monotherapy achieved a marked response (76.3% decrease in serum CEA levels) in this case than that expected in the management of metastatic MPBC.
In conclusion, serum CEA elevation may be associated with MPBC and MPBC should be reported in the pathology report, regardless of the size of the MPC component. Radical cystectomy may be offered rather than BCG treatment to patients with MPBC. If radical cystectomy cannot be performed, the possibility of metastasis should always be considered, even if no bladder recurrence is observed in the follow-up. Gemcitabine monotherapy can be administered in these patients, and serum CEA level may be used as a parameter for evaluating CT response.
ACKNOWLEDGMENT:
The author would like to thank the entire staff of the Department of Urology and Pathology in Sultan Abdul Hamid Han Education and Research Hospital, Istanbul, Turkey.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
CE: Contributed to the conception and design of the case report.
CE, SA: Collection of data.
CE, NK: Revision of the manuscript.
OY, NK: Preparing figures and performing he last evaluation.
All the authors read and approved the final manuscript.
REFERENCES
- Spaliviero M, Dalbagni G, Bochner BH, Poon BY, Huang H, Al-Ahmadie HA, et al. Clinical outcome of patients with T1 micropapillary urothelial carcinoma of the bladder. J Urol 2014; 192(3):702-7. doi: 10.1016/j.juro.2014.02.2565.
- Yang J, Tang A, Ma J, Sun X, Ming L. The reference intervals for CA125, CA15-3, CA19-9, CA72-4, AFP, CEA, NSE and CYFRA21-1. Scand J Clin Lab Invest 2019; 79(1-2):71-4. doi: 10.1080/00365513.2018.1555855.
- Koizumi K, Irie K, Yokoyama H, Hosaka K, Takezaki T, Koyama T. [Case report: Lymph node metastasis of micropapillary bladder tumor that completely responded to radiation therapy]. Nihon Hinyokika Gakkai Zasshi 2008; 99(5):652-5. doi: 10.5980/jpnjurol1989.99.652.
- Ohtsuki Y, Ochi K, Okada Y, Kato M, Lee GH, Furihata M. Micropapillary component of urothelial carcinoma detected in transurethral resection of bladder tumor (TUR-BT) tissues: A case report. Med Mol Morphol 2008; 41(2):113-6. doi: 10.1007/s00795-007-0392-5.
- Haholu A, Ekinci O, Ateş F, Yigit N, Baykal K, Baloglu H. Mesanenin mikropapiller tip degisici epitel hucreli karsinomu klinik, histolojik ve immunohistokimyasal ozellikler: Olgu Sunumu. Med J Bakirkoy 2011; 7(4).
- Kamat AM, Dinney CP, Gee JR, Grossman HB, Siefker-Radtke AO, Tamboli P, et al. Micropapillary bladder cancer: A review of the University of Texas M. D. Anderson cancer center experience with 100 consecutive patients. Cancer 2007; 110(1): 62-7. doi: 10.1002/cncr.22756.
- Willis DL, Fernandez MI, Dickstein RJ, Parikh S, Shah JB, Pisters LL, et al. Clinical outcomes of cT1 micropapillary bladder cancer. J Urol 2015; 193(4):1129-34. doi: 10. 1016/j.juro.2014.09.092.
- Sui W, Matulay JT, James MB, Onyeji IC, Theofanides MC, RoyChoudhury A, et al. Micropapillary bladder cancer: Insights from the national cancer database. Bladder Cancer 2016; 2(4):415-23. doi: 10.3233/BLC-160066.
- Kyoda Y, Kunishima Y, Fukuta F. [Experience with gemcitabine monotherapy in three patients with metastatic urothelial carcinoma]. Nihon Hinyokika Gakkai Zasshi 2011; 102(5):701-4. doi: 10.5980/jpnjurol.102.701.
- Albers P, Siener R, Hartlein M, Fallahi M, Haeutle D, Perabo FG, et al. Gemcitabine monotherapy as second-line treatment in cisplatin-refractory transitional cell carcinoma prognostic factors for response and improvement of quality of life. Onkologie 2002; 25(1): 47-52. doi: 10.1159/000 055202.