Acute Oral Mucositis During Hypo-Fractionated Radiation in Squamous Cell Carcinoma of Oral Cavity
By Agha Muhammad Hammad, Sehrish Abrar, Rabia Tahseen, Bilal Mazhar Qureshi, Asim Hafiz, Ahmed Nadeem AbbasiAffiliations
doi: 10.29271/jcpsp.2023.12.1460ABSTRACT
Oral mucositis remains a concern in the treatment of head and neck malignancies. This small study included 11 patients treated by hypo-fractionated radiotherapy and assessed for oral mucositis. All patients received a radiation dose of 55 Gy in 20 fractions (2.75 Gy/fraction). At the end of the first week of radiation, three patients had Grade I oral mucositis. During the last week of radiation, most of the patients developed Grade II and III mucositis, 7 (64%) and 4 (36%), respectively. At one month follow-up, 5 (46%) of them had Grade I, while 2 (18%) had developed Grade II mucositis. At three months, 2 (18%) had Grade I mucositis, and none of the patients showed Grade II/III oral mucositis. Grade II oral mucositis was the most common grade found mainly in the last week of radiation therapy. None had Grade IV oral mucositis.
Key Words: Acute oral mucositis, Hypo-fractioned radiation, Oral carcinoma.
Radiation therapy is of paramount importance in the treat-ment of oral cavity cancers in the radical and adjuvant settings to achieve local control rates along with concurrent chemo-therapy.1 Hypo-fractionated radiation therapy gives high local control rate in locally advanced head and neck cancers in both radical and palliative arena, making it a significantly altered fractionation schedule and cost-effective approach for the patient of head and neck cancers as it completes the treatment in lesser duration.2 However, there is a list of side effects associated with radiation therapy in the face and neck region. One of the most important acute side effects is oral mucositis with primary symptoms of mouth pain, mouth dryness, eating difficulties, and swallowing which results in treatment breaks and causes loss of local control rate by 1.4% per day because of the interruption during planned treatment in radiation therapy.3 Previous studies have shown that oral mucositis develops in 80-83% of patients receiving radiation therapy to head and neck with 19%, 35%, and 28% developing mild, moderate, and severe mucositis, respectively with conventional fractionation.4 For the hypo-fractioned schedule, Grade III and IV mucositis were experienced in 78% and 4% of patients, respectively.4
Hypo-fractionated radiotherapy 55 Gy in 20 fractions of 2.75 Gy was delivered for head and neck tumours at the Radiation Oncology Department, The Aga Khan University Hospital, Karachi. WHO scale was used for assessing the frequency and severity of mucositis after radiotherapy. Treatment records of 11 eligible patients with histopathology proven, squamous cell carcinoma of the oral cavity were retrospectively reviewed. One patient (9%) at Stage I, five patients (46%) at Stage II, three patients (27%) at Stage III, and two patients (18%) at Stage IV according to the American Joint Committee on Cancer (AJCC) 8th Edition, were treated with hypo-fractionated radiation schedule with or without concurrent chemotherapy after surgery. The study was conducted from January 2019 to December 2020 at the Oncology Department of Aga Khan University Hospital in Pakistan All patients who had received this regimen, had Eastern Cooperative Oncology Group (ECOG) performance status 0-2. Any patients who had metastatic disease or had received prior chemotherapy radiation therapy with conventional fractionation were excluded from the analysis. The demographic data of the subjects are shown in Table I.
At the end of the first week of radiation, 8 patients (72%) did not have any grade of mucositis. Only 3 (28%) patients were found to have mucositis, all with the Grade I severity. During the last week of radiation, all eleven patients (100%) had acute oral mucositis. The severity ranged between Grade II and III oral mucositis. Grade II mucositis was the most frequent (n= 7, 64%), while 4 (36%) had Grade III oral mucositis. After the completion of radiation therapy, follow-up was done after one month to assess recovery from acute oral reaction. Oral mucositis was still positive in 8 patients (73%). Among those in whom oral mucositis persisted, 5 (46%) had Grade I, while 2 (18%) had Grade II mucositis. Only one (9%) patient had Grade III mucositis. At three months follow-up, 2 (18%) had Grade I mucositis, none of the patients showed Grade II/III oral mucositis, and all patients started oral feeding. All patients (100%) developed acute oral mucositis during the radiation therapy. Grade II oral mucositis was the most common grade found mainly in the last week of radiation therapy. None of the patients developed Grade IV oral mucositis during radiation. The frequency and severity of acute oral mucositis are shown in Figure 1. All the patients who experienced Grade III oral mucositis had Stage III/ IV disease, a large volume were treated (oral cavity along with whole ipsilateral neck) and received weekly cisplatin.
Table I: Demographics of included participants.
|
Characteristics |
n (%) |
|
Gender Male Female |
9 (82%) 2 (18%) |
|
Sites Buccal mucosa Upper alveolus Retromolar trigone |
9 (82%) 1 (9%) 1 (9% |
|
Stage Stage I Stage II Stage III Stage IV |
1 (9%) 5 (46%) 3 (27%) 2 (18%) |
|
Surgery |
11 (100%) |
|
Techniques 3DCRT |
11 (100%) |
|
Chemotherapy Yes No |
6 (55%) 5 (45%) |
|
Intent Curative Palliative |
8 (73%) 3 (27%) |
|
Addiction Yes No |
8 (73%) 3 (27%) |
|
Education status Primary education Matriculation Graduation degree Master’s degree |
4 (37%) 2 (18%) 2 (18%) 3 (27%) |
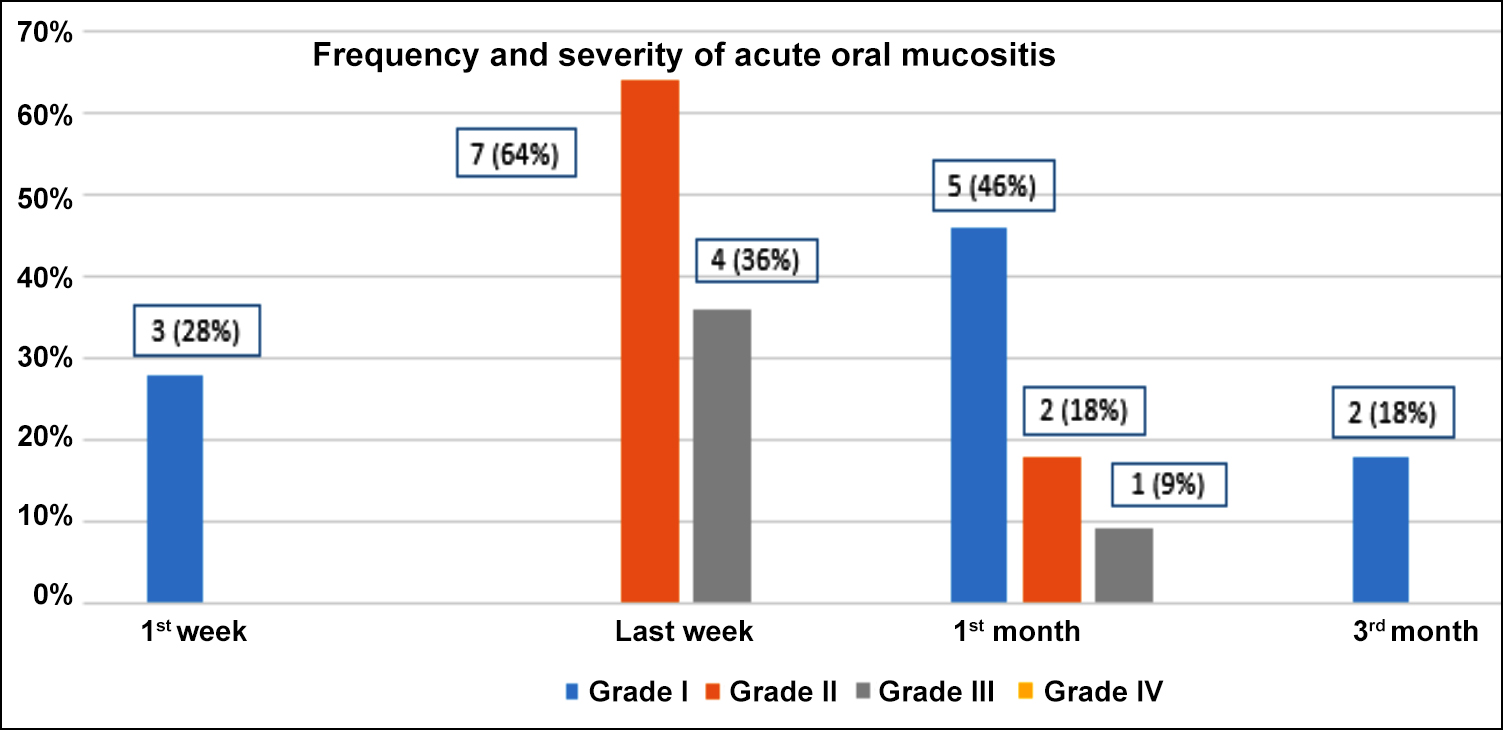 Figure 1: Frequency and severity of acute oral mucositis.
Figure 1: Frequency and severity of acute oral mucositis.
The results of this study are reflective of the previous published studies. Jacinto et al. reported overall 30-40% Grade III dermatitis with hypo-fractionated regimen (55Gy/20 fx) along with chemotherapy.5 In this study, eight patients (40%) experienced Grade III mucositis during the last week of treatment. No Grade IV dermatitis occurred and all patients had complete resolution of the mucositis and dermatitis up to one month after the treatment.5
An important message from this study is the development of Grade I oral mucositis which is observed in one-third of patients, rendering the importance of early assessment for hypo-fractionated schedule patients. The average time of initiation of acute oral mucositis is between 5 to 14 days with conventional fractionation, but in this study, dose per fraction was an important factor that explained the higher and early incidence of Grade I oral mucositis. In this study, during radiation, all patients had oral mucositis, mostly Grade II and III. All the published literature focused on Grade III and IV toxicity, as these grades hinder oral feeding and hamper the quality of life, resulting in treatment breaks and loss of local control by 1.4% on a daily cumulative basis. Grade III and IV oral mucositis is well reported in this schedule. In the group, none of the patients developed Grade IV mucositis; and at three months follow-up, none of the patients experienced Grade III/IV mucositis. Moreover, none of the patients had a gap during the treatment because of oral mucositis.
A recently published Indian study reported treatment gaps in up to 39% patients due to extreme grades of mucositis.6 The present authors observed that the reason for not observing the treatment gaps in these patients is the insertion of feeding tube before starting the treatment to avoid nutritional deficiency. None of the altered scheduled studies published up till now had reported a trend of development oral mucositis. This study identified frequency and trend of developing severity of mucositis during RT. The main limitations of this study were limited number of patients and the retrospective study design.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
AMH: Substantial contributions to the conception and design of the work, drafting the work and revising it critically.
RT, SA: Literature search and manuscript writing.
BMQ, ANA: Final approval of the version to be published, drafting the work or revising it critically for important intellectual content.
AH: Review of the manuscript and verification of references.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Alterio D, Marvaso G, Ferrari A, Volpe S, Orecchia R, Jereczek-Fossa BA. Modern radiotherapy for head and neck cancer. Semin Oncol 2019; 46(3):233-45. doi: 10.1053/j. seminoncol.2019.07.002.
- Vreugdenhil M, Fong C, Sanghera P, Hartley A, Dunn J, Mehanna H. Hypofractionated chemoradiation for head and cancer: Data from the PET NECK trial. Oral Oncol 2021; 113:105112. doi: 10.1016/j.oraloncology.2020.105112.
- Russo G, Haddad R, Posner M, Machtay M. Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist 2008; 13(8):886-98. doi: 10.1634/ theoncologist.2008-0024.
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: A systematic literature review. Radiother Oncol 2003; 66(3):253-62. doi: 10.1016/s0167-8140(02)00404-8.
- Jacinto AA, Batalha-Filho ES, Viana LD, De Marchi P, Capuzzo RD, Gama RR, et al. Feasibility of concomitant cisplatin with hypofractionated radiotherapy for locally advanced head and neck squamous cell carcinoma. BMC Cancer 2018; 18(1):1-9. doi: 10.1186/s12885-018-4893-5.
- Rangineni S, Lahiri D, Misra B, Maji T, Roy S, Ray DK, et al. Hypofractionated intensity-modulated radiation therapy with concurrent cisplatin in locally advanced oropharyngeal cancer: Feasibility experience from a government cancer centre of Eastern India in a resource-constrained setting. J Radiother Pract 2020; 19(1):59-64. doi: 10.1017/S146039 6919000323.