A Meta-analysis of the Risk Factors for Stroke-associated Pneumonia
By Shou-Ye Zhang, Jing Huang, Xiao-Ling ZhouAffiliations
doi: 10.29271/jcpsp.2023.07.799ABSTRACT
This study aimed to conduct a meta-analysis to assess the possible risk factors of stroke-associated pneumonia (SAP). A comprehensive search of PubMed, Medline, and Cochrane Library was gathered from 2000 to April 2022. A case-control study evaluating the risk factors of SAP was selected. The major finding of this study was that dysphagia, atrial fibrillation, gender, diabetes mellitus, and hypertension were risk factors that determine the development of SAP. The random-effects strategy was used to highlight study-specific outcomes. Only 14 papers out of 651 met the criteria for inclusion and were included in the study. The quality of this study was generally excellent. Risk factors associated with SAP were gender (pooled OR 1.48,95% CI 1.18-1.85), dysphagia (pooled OR 2.61, 95% CI 1.97-3.46), atrial fibrillation (pooled OR 2.08, 95% CI 1.68-2.57), diabetes mellitus (pooled OR 1.32, 95% CI 1.17-1.49), hypertension (pooled OR 1.19, 95% CI 1.05-1.34). This research is crucial because some risk factors are easily recognised, and patients with one or more of these risk factors were developing SAP. Disorders such as dysphagia, atrial fibrillation, diabetes, and hypertension should be addressed and managed to reduce the incidence of SAP conundrums.
Key Words: Ischemic stroke, Pneumonia, Risk factor.
INTRODUCTION
Cardiovascular disorders, fever, venous thromboembolism, dysphagia, pain, depression, pneumonia, and incontinence are all frequent comorbidities in stroke patients, with stroke-related pneumonia being the most common. According to the literature, the incidence rate of SAP varied between 5% and 30% in different countries.1 Despite the fact that severe strokes cause early mortality, pneumonia remains the leading cause of death following an acute stroke, regardless of hospitalisation.2 Yuan et al., conducted a meta-analysis on the risk factors for stroke-associated pneumonia (SAP).3 However, the great majority of studies evaluated only provided crude ORs (univariate regression analysis), despite the large potential for confounding by age, gender, and other associated factors, adjusted ORs are more reliable. As a result, a comprehensive systematic review and meta-analysis of the prevalence of SAP were performed in order to study the risk factors, and these results are anticipated to be beneficial to health professionals working with SAP patients.
METHODOLOGY
A comprehensive search was undertaken in the PubMed, Cochrane databases, and Embase from 2000 to April 2022. Using the following key phrases ‘Ischemic Stroke’, ‘Pneumonia’ ‘no COVID-19’, a systematic review of the literature was carried out with the goal to identify articles. Non-English language literature, unrelated articles, and nonrandomised experimental designs were disqualified. Six hundred and fifty-one papers were assessed for the literature review, with data from 14 of them being collected. Figure 1 depicts the article evaluation process. Each study was assessed by two independent investigators. The Newcastle-Ottawa Scale (NOS), a quality assessment tool, was used to evaluate the quality of the literature. Those with five stars were selected for the study. A group discussion was used to resolve any disputes among the reviewers.
Data from the 14 case-control studies were analysed by two scientists (Jing Huang and Shou-Ye Zhang). Raw data such as author, year, study type, number of patients in control and disease, article impact factors, odds ratio (OR), and 95 percent confidence interval (CI) for each included study were extracted.
The chi-square test for heterogeneity was used in the statistical analysis, which was done using Review Manager Software (RevMan 5.4). Utilising a Random-effects model to assess the pooled impact size. When I2 values above 75%, which is regarded to indicate considerable heterogeneity. ORs with 95 percent confidence intervals and a p-value of less than or equal to 0.05 were considered statistically significant.
 Figure 1: Search results and selection of articles.
Figure 1: Search results and selection of articles.
HR = Hazard ratio; RR = Relative risk.
The sensitivity analysis was performed by removing each trial one at a time, and the substantial pooled OR and 95 percent CI remained unchanged, indicating that the meta-analysis' conclusions were reliable. Because the meta-analysis included a small number of studies, no funnel plots were utilised to examine publication bias for specific risk factors.
RESULTS
Only patients who satisfied the inclusion criteria were included in the meta-analysis. The full selection technique is depicted in detail in Figure 1.
Although single-effect estimates were seldom reported, gender was frequently used as an adjustment factor in publications. Five studies looked at the magnitude of the impacts by gender.4-8 It has long been suspected that men are more susceptible to SAP than women (pooled OR 1.48, 95% CI 1.18-1.85). Due to higher heterogeneity, one paper was omitted from the meta-analysis (Figure 2).9
One out of the ten studies5 that looked into dysphagia as a risk factor for SAP was taken out, and the result is shown in Figure 3.4,6,10-15 Due to the inadequate sample size and higher OR than other studies, this article was disregarded. Although the reported discovery had only a modest impact on the overall research findings, it would be of interest to the Chinese population.
The consequences of atrial fibrillation on SAP are seen in Figure 4. Based on nine studies that looked at the impact of atrial fibrillation, we reported the pooled OR and 95 percent confidence interval, as well as a heterogeneity test.4-8,12,13,15,16
These findings are based on the results of six papers. Figure 5 depicts the pooled ORs.5,6,9,12,16,17
There are a total of six literature that met the criteria for inclusion in this variable. Figure 6 shows the summaries of the result.5,6,9,10,12,16
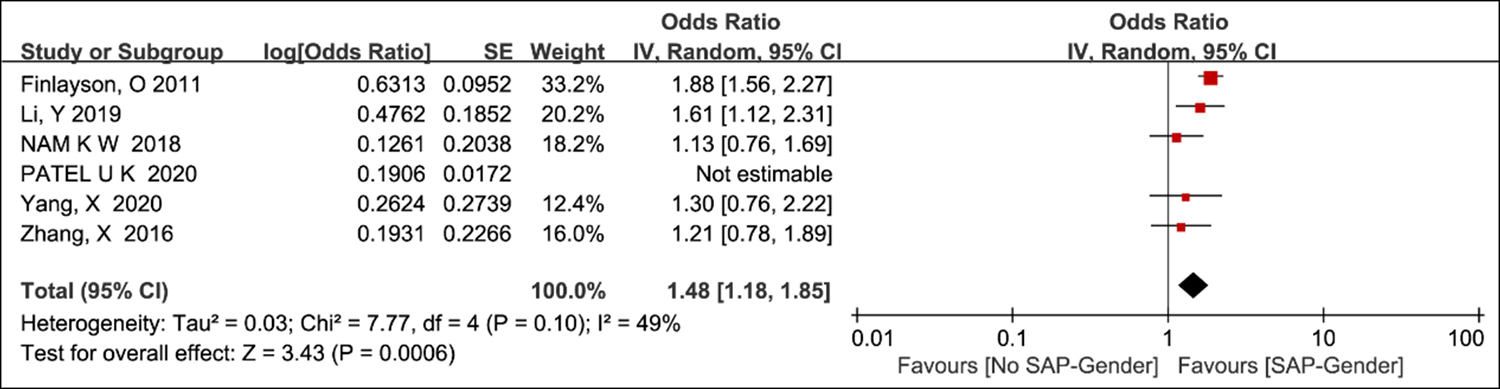 Figure 2: Odds ratio plot for risk factor gender.
Figure 2: Odds ratio plot for risk factor gender.
 Figure 3: Odds ratio plot for risk factor dysphagia.
Figure 3: Odds ratio plot for risk factor dysphagia.
 Figure 4: Odds ratio plot for risk factor atrial fibrillation.
Figure 4: Odds ratio plot for risk factor atrial fibrillation.
 Figure 5: Odds ratio plot for risk factor diabetes mellitus.
Figure 5: Odds ratio plot for risk factor diabetes mellitus.
 Figure 6: Odds ratio plot for risk factor hypertension
Figure 6: Odds ratio plot for risk factor hypertension
DISCUSSION
This thorough study and meta-analysis demonstrated that dysphagia, atrial fibrillation, gender, diabetes mellitus, and hypertension were all risk factors for SAP. Hoffmann developed the A2DS2 scoring system, which included the risk factors described above.18 Several papers suggested that age5,8 and stroke severity6-8 might be risk factors for SAP, but they were unable to reach conclusive results. These studies lacked a unified standard. Although age and the severity of the stroke were not taken into account in investigation, two key risk factors were identified as diabetes and hypertension.
Ischemic stroke has been linked to racial and gender inequalities.19 A large sample study suggested that females consistently displayed a lower health-related quality of life than males after stroke. This might be attributed to women longevity, post-stroke depression, and more severe strokes.20 Similarly, the influence of gender on stroke severity and outcomes has been established. Females appeared to have a worse prognosis, which was often connected to more severe symptoms at the beginning and a delay in treatment.21 Although several researchers have demonstrated that females had a worse prognosis after a cerebral infarction, data are not contradictory. This might be because males smoke more and have more pre-existing pulmonary illnesses than females.22,23
Dysphagia affects around one-third of stroke patients, with 20% suffering from aspiration pneumonia.24 Stroke-associated pneumonia is mainly driven by dysphagia and aspiration pneumonia after a stroke.25 As a consequence, current research shows that early dysphagia screening might help lower stroke-related pneumonia rates and could be a method for improving stroke outcomes, especially in people who have recently had an ischemic stroke.12 According to the hypothesis, dysphagia caused by a stroke might lead to aspiration of oropharyngeal or stomach contents, which may well lead to pneumonia.26
Atrial fibrillation (AF) is a major risk factor for ischemic stroke, accounting for 45% of all cardioembolic strokes.27 Furthermore, AF is a modifiable risk factor for ischemic stroke; approximately 20% and 30% of patients with ischemic stroke had a diagnosis of AF prior to, during, or after the stroke episodes,28 demonstrating the need for active management of AF for stroke prevention. According to multiple studies, Pneumonia is significantly more prevalent in Ischemic Stroke patients with AF than in those without AF (9.8% vs. 5.3%).29 A body of research shows scant evidence of the relationship between AF and SAP. Investigating if there is anything else that may explain this finding, such as atrial abnormalities, which can lead to reduced cardiac output and lung congestion, increasing the risk of pulmonary infection.30 Because the subtypes of distinct AF were not completely examined, the results should be interpreted with caution. This will necessitate more study to better understand the impact of a variety of processes.
Diabetes and its complications have been recognised as a severe public health hazard as the incidence of diabetes has steadily climbed.31 Microvascular problems of diabetes imperil the eye, foot, and kidney. In patients with diabetes, decreased pulmonary function and pulmonary microangiopathy have been identified.32 According to Jette’s findings from a large population-based case-control research, diabetes and HbA1C levels are risk factors for pneumonia-related hospitalisation.33
In comparison to the other four risk factors, the significance of these differences in OR for hypertension appears to be modest. Despite the fact that patients have been diagnosed with hypertension, it was unable to obtain information on their blood pressure control, such as the length of their disease, their blood pressure readings, and the drugs they were taking. Following up on Kenneth's discovery that both calcium channel blockers and blockers were connected to an increased risk of pneumonia. As a result, the discovery of hypertension as a SAP risk factor should be viewed with caution.34 A 24-hour ambulatory blood pressure measurement is the gold standard for blood pressure measurement. If it were able to collect these details from the authors and prove the point, targeting hypertension as a treatment for SAP might be an innovative approach, however, it was not performed in every study.
CONCLUSION
Patients with dysphagia, atrial fibrillation, diabetes, and hypertension should be constantly monitored. However, findings may exaggerate the link between hypertension and SAP. More investigation is necessary to uncover these connections, particularly in the areas of hypertension, age, and stroke severity.
ACKNOWLEDGEMENTS:
This study received no particular support from governmental, private, or non-profit funding bodies.
DISCLOSURE:
All authors report no conflicts of interest in this work.
AUTHORS’ CONTRIBUTION:
SYZ: Data curation and writing the original draft.
XLZ: Visualisation and investigation.
JH: Supervision and writing review editing.
REFERENCES
- Xu J and Yang Z. Risk factors and pathogenic micro-organism characteristics for pneumonia in convalescent patients with stroke: A retrospective study of 380 patients from a rehabilitation hospital. J Stroke Cerebrovas Dis 2020; 29(8);104955. doi: 10.1016/j.jstrokecerebrovasdis. 2020.104955.
- Prass K, Meisel C, Hoflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med 2003; 198(5): 725-36. 2003/08/27. doi: 10.1084/jem.20021098.
- Yuan MZ, Li F, Tian X, Wang W, Jia M, Wang XF, et al. Risk factors for lung infection in stroke patients: A meta-analysis of observational studies. Expert Rev Anti Infect Ther 2015; 13(10):1289-98. 2015/09/12. doi: 10.1586/14787210.2015. 1085302.
- Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G, et al. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurol 2011; 77(14):1338-45. doi: 10.1212/WNL.0b013e31823 152b1.
- Zhang X, Yu S, Wei L, Ye R, Lin M, Li X, et al. The A2DS2 score as a predictor of pneumonia and in-hospital death after acute ischemic stroke in Chinese populations. PLoS ONE 2016; 11(3):e0150298. doi: 10.1371/journal.pone. 0150298.
- Nam KW, Kim TJ, Lee JS, Kwon HM, Lee YS, Ko SB, et al. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke 2018; 49(8):1886-92. doi: 10.1161/STROKEAHA.118.021228.
- Li Y, Zhang Y, Ma L, Niu X, Chang J. Risk of stroke-associated pneumonia during hospitalization: Predictive ability of combined A2DS2 score and hyperglycemia. BMC Neurol 2019; 19(1):298. doi: 10.1186/s12883-019-1497-x.
- Yang X, Wang L, Zheng L, Wu J, Liu J, Hao Z, et al. Serum albumin as a potential predictor of pneumonia after an acute ischemic stroke. Curr Neurovas Res 2020; 17(4):385-93. Article. doi: 10.2174/156720261766620051 4120641.
- Patel UK, Kodumuri N, Dave M, Lekshminarayanan A, Khan N, Kavi T, et al. Stroke-Associated Pneumonia: A retrospective study of risk factors and outcomes. Neurologist 2020; 25(3):39-48. doi: 10.1097/NRL.00000 00000000269.
- Ishigami K, Okuro M, Koizumi Y, Satoh K, Iritani O, Yano H, et al. Association of severe hypertension with pneumonia in elderly patients with acute ischemic stroke. Hypertens Res 2012; 35(6): 648-53. 2012/02/10. doi: 10.1038/hr.2012.7.
- Herzig SJ, Doughty C, Lahoti S, Marchina S, Sanan N, Sanan W, et al. Acid-suppressive medication use in acute stroke and hospital-acquired pneumonia. Ann Neurol 2014; 76(5):712-18. doi: 10.1002/ana.24262.
- Al-Khaled M, Matthis C, Binder A, Mudter J, Schattschneider J, Pulkowski U, et al. Dysphagia in patients with acute ischemic stroke: Early dysphagia screening may reduce stroke-related pneumonia and improve stroke outcomes. Cerebrovascular Diseases 2016; 42(1-2):81-89. doi: 10. 1159/000445299.
- Hoffmann S, Harms H, Ulm L, Nabavi DG, Mackert BM, Schmehl I, et al. Stroke-induced immunodepression and dysphagia independently predict stroke-associated pneumonia - The PREDICT study. J Cereb Blood Flow Metab 2017; 37(12):3671-82. doi. 10.1177/0271678x16671964.
- Huang GQ, Cheng HR, Wu YM, Cheng QQ, Wang YM, Fu JL, et al. Reduced vitamin D levels are associated with stroke-associated pneumonia in patients with acute ischemic stroke. Clin Interv Aging 2019; 14:2305-2314. doi: 10.2147/CIA.S230255.
- Yuan M, Li Q, Zhang R, Zhang W, Zou N, Qin X, et al. Risk factors for and impact of poststroke pneumonia in patients with acute ischemic stroke. Medicine 2021; 100(12): e25213. doi: 10.1097/MD.0000000000025213.
- Ovbiagele B, Hills NK, Saver JL, Johnston SC. Frequency and determinants of pneumonia and urinary tract infection during stroke hospitalization. J Stroke Cerebrovasc Dis 2006; 15(5): 209-213. 2007/10/02. doi.10.1016/j.jstrok ecerebrovasdis.2006.05.004.
- Han. Prognostic risk factors for stroke-associated pneumonia. Neural Regeneration Research 2010; 5:1592-5. doi.10.3969/j.issn.1673-5374.2010.20.013.
- Hoffmann S, Malzahn U, Harms H, Koennecke HC, Berger K, Kalic M, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke 2012; 43(10):2617-23. 2012/07/17. doi. 10.1161/strokeaha.112. 653055.
- Howard VJ, Madsen TE, Kleindorfer DO, Judd SE, Rhodes JD, Soliman EZ, et al. Sex and race differences in the association of incident ischemic stroke with risk factors. JAMA Neurol 2019; 76(2): 179-86. 2018/12/12. doi.10. 1001/jamaneurol.2018.3862.
- Phan HT, Blizzard CL, Reeves MJ, Thrift AG, Cadilhac DA, Sturm J, et al. Sex differences in long-term quality of life among survivors after stroke in the instruct. Stroke 2019; 50:2299-2306. 2019/08/16. DOI: 10.1161/strokeaha.118. 024437.
- Falsetti L, Viticchi G, Buratti L, Balucani C, Marra AM, Silvestrini M, et al. From head to toe: Sex and gender differences in the treatment of ischemic cerebral disease. Pharmacol Res 2017; 121:240-50. 2017/05/17. doi.10. 1016/j.phrs.2017.05.006.
- Jha P, Ranson MK, Nguyen SN, Yach D. Estimates of global and regional smoking prevalence in 1995, by age and sex. Am J Public Health 2002; 92(6):1002-6. 2002/05/31. DOI: 10.2105/ajph.92.6.1002. doi: 10.2105/ajph.92.6.1002.
- de Miguel-Yanes JM, Lopez-de-Andres A, Jiménez-Garcia R, Hernandez-Barrera V, De Miguel-Diez J, Carabantes-Alarcon D, et al. Incidence, outcomes and sex-related disparities in pneumonia: A matched-pair analysis with data from spanish hospitals (2016-2019). J Clin Med 2021; 10(19): 4339. doi: 10.3390/jcm10194339.
- Aviv JE, Sacco RL, Thomson J, Diamond B, Martin JH, Close LG. Silent laryngopharyngeal sensory deficits after stroke. Ann Otol Rhinol Laryngol 1997; 106(2):87-93. doi: 10. 1177/000348949710600201.
- Hilker R, Poetter C, Findeisen N, Sobesky J, Jacobs A, Neveling M, et al. Nosocomial pneumonia after acute stroke: Implications for neurological intensive care medicine. Stroke 2003; 34(4):975-81. doi: 10.1161/01. Str.0000063373.70993.Cd.
- Westendorp WF, Nederkoorn PJ, Vermeij JD, Dijkgraaf MG, van de Beek D. Post-stroke infection: A systematic review and meta-analysis. BMC Neurol 2011; 11:110. doi: 10. 1186/1471-2377-11-110.
- Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014; 45(5):1545-88. doi: 10.1161/01. str.0000442009.06663.48.
- Hsu CY, Singer DE, Kamel H, Wu YL, Chen PC, Lee JD, et al. Unrecognised history of transient atrial fibrillation at the time of discharge from an index stroke hospitalisation is associated with increased recurrent stroke risk. J Stroke 2019; 21(2):190-4. doi: 10.5853/jos.2018.03265.
- Keller K, Hobohm L, Wenzel P, Münzel T, Espinola-Klein C, Ostad MA, et al. Impact of atrial fibrillation/flutter on the in-hospital mortality of ischemic stroke patients. Heart Rhythm 2020; 17(3):383-90. doi: 10.1016/j.hrthm.2019. 10.001.
- Zhu J, Zhang X, Shi G, Yi K, Tan X. Atrial fibrillation is an independent risk factor for hospital-acquired pneumonia. PLoS One 2015; 10(7):e0131782. doi: 10.1371/journal. pone.0131782.
- Sachan R, Kundu A, Dey P, Son JY, Seok Kim K, et al. Dendropanax morbifera protects against renal fibrosis in streptozotocin-induced diabetic Rats. Antioxidants (Basel) 2020; 9(1):84. doi: 10.3390/antiox9010084.
- Koziel H and Koziel MJ. Pulmonary complications of diabetes mellitus. Pneumonia. Infect Dis Clin North Am 1995; 9(1):65-96. doi: 10.1016/S0891-5520(20)30641-3.
- Kornum JB, Thomsen RW, Riis A, Lervang HH, Schønheyder HC, Sorensen HT, et al. Diabetes, glycemic control, and risk of hospitalisation with pneumonia: A population-based case-control study. Diabetes Care 2008; 31(8):1541-5. doi: 10.2337/dc08-0138.
- Mukamal KJ, Ghimire S, Pandey R, Ellen S, Meara O, Shiva G. Antihypertensive medications and risk of community-acquired pneumonia. J Hypertens 2010; 28(2):401-5. doi: 10.1097/HJH.0b013e3283330948.