A Difficult Route for Percutaneous Coronary Intervention: Single Coronary Artery Anomaly
By Kerim Esenboga, Emir Baskovski, Nil Ozyunku, Eralp TutarAffiliations
doi: 10.29271/jcpsp.2022.04.3ABSTRACT
Coronary artery anomalies (CAA) are frequently asymptomatic clinical entities, associated with variable prognosis. Single coronary artery anomaly is a rare type of coronary anatomy anomaly with little data in the literature. Although prognostic data are not clear, it is thought that the course of anomalous artery is the most important determinant. Percutaneous revascularisation of concomitant atherosclerotic disease in anomalous coronary arteries may be technically challenging. In this report, we present a case of a single coronary artery undergoing percutaneous revascularisation with an alternative technique of advancing a guidewire into the anomalous left main coronary artery and then crossing the culprit lesion in the left anterior descending artery. This report highlights the importance of catheter stabilisation during percutaneous revascularisation with a simple yet very practical solution.
Key Words: Coronary angiography, Coronary vessel anomalies, Myocardial infarction, Percutaneous coronary intervention.
INTRODUCTION
Coronary artery anomalies (CAA) are a heterogeneous group of clinical entities that are usually incidentally detected. While most are asymptomatic, they can present with chest pain, dyspnea and more rarely as sudden cardiac death (SCD).1 In this report, we describe a case of the single coronary artery (SCA), presenting with non-ST elevated myocardial infarction (NSTEMI). The percutaneous revascularisation in anomalous coronary arteries can be challenging; and this case report proposes simple, yet very practical solution to fine-guide manipulation.
CASE REPORT
A 60-year woman presented to the Emergency Department with chest pain lasting six hours. The patient’s clinical history included coronary artery disease, diabetes mellitus treated with oral antidiabetics and hypertension. Four years prior to this presentation, the patient had undergone successful percutaneous coronary intervention (PCI) for medically resistant angina, and was asymptomatic thereafter. The electrocardiogram (ECG) showed no signs of myocardial ischemia; however, serial troponin measurement revealed a progressive increase that was consistent with NSTEMI. An echocardiogram displayed mild hypokinesia of the anterior left ventricular wall.
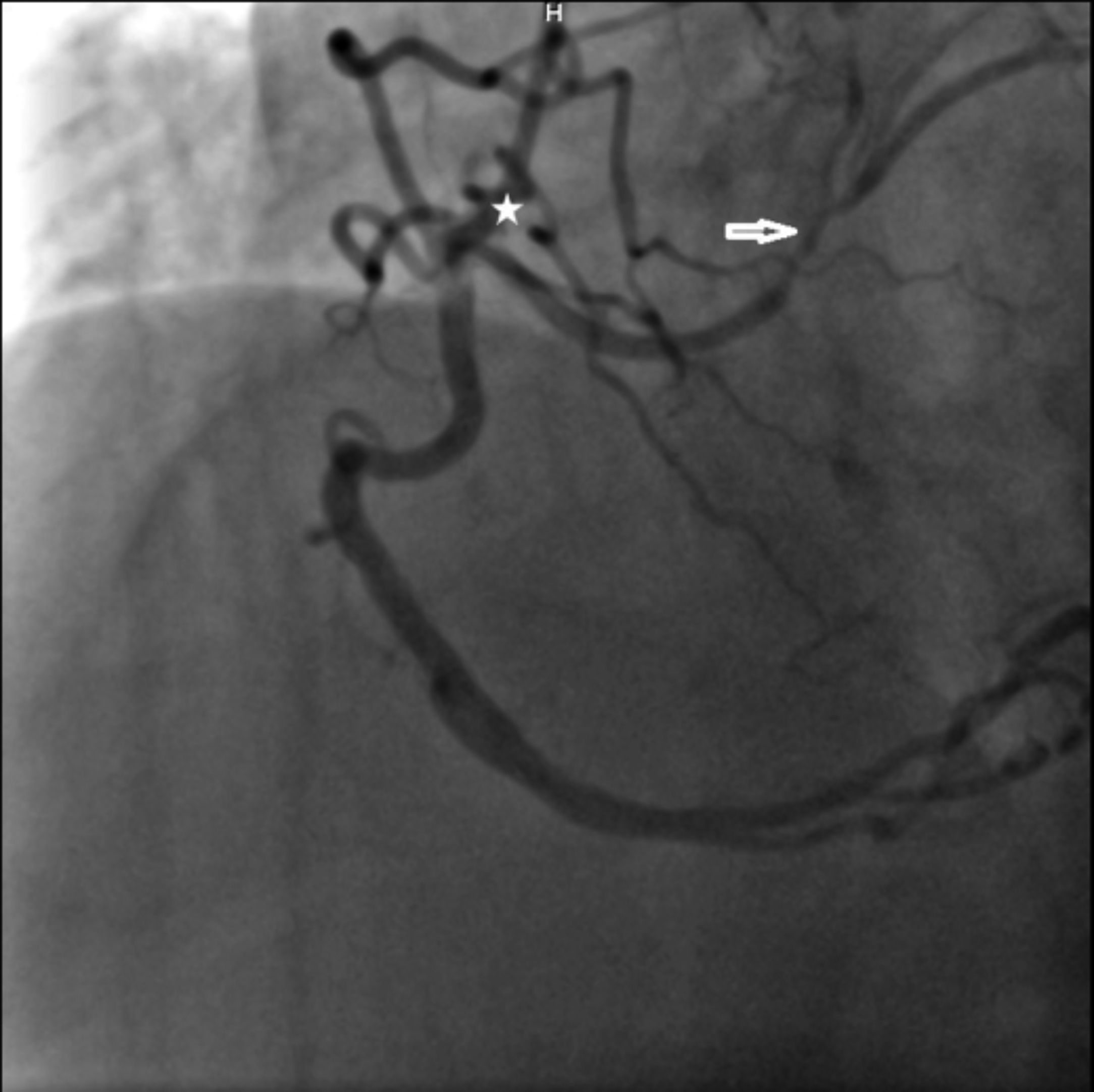 Figure 1: An anomalous short left coronary artery originating from the right coronary artery can be observed. Circumflex artery is non-dominant (*). Angiographically significant stenosis is present in the left anterior descending artery (arrow).
Figure 1: An anomalous short left coronary artery originating from the right coronary artery can be observed. Circumflex artery is non-dominant (*). Angiographically significant stenosis is present in the left anterior descending artery (arrow).
The patient was hospitalised and medical treatment was initiated. Coronary angiography revealed a SCA with short left main coronary artery (LMCA), originating from the ostial right coronary artery (RCA), then bifurcating into the left anterior descending (LAD) and non-dominant left circumflex (LCx) arteries (Figure 1). While a patent coronary stent was present in the mid RCA, an angiographically significant stenosis was visible in the LAD before the first diagonal branch. After reviewing coronary anatomy and clinical data, ad-hoc PCI was planned. Initially, a Judkins right (JR) guiding catheter was selected for guidewire advancement; however, the guidewire tip could not be advanced to the LMCA. Subsequent advancement of the guidewire was attempted using Amplatz left (AL) 2, Shepard’s crook, and finally Amplatz right (AR) 2 catheters; but because the catheter tip-to-LMCA angle was not favourable, the guidewire tip kept slipping into the RCA. Guide tip manipulation to improve the angle could not be achieved due to disengagement of the tip from the RCA. To stabilise the AR2 catheter and allow tip manipulation, the guidewire was advanced into the distal RCA. Thereafter, with a slight clockwise rotation, the catheter tip was pointed toward the LMCA. Finally, a second guidewire was advanced to the LMCA, and thereafter the LAD artery. Following predilation, a drug-eluting stent was implanted into the LAD lesion (Figure 2). Twenty-four hours later, the patient was discharged with appropriate medical therapy. One month following the initial event, an exercise stress myocardial perfusion imaging revealed no objective evidence of myocardial ischemia.
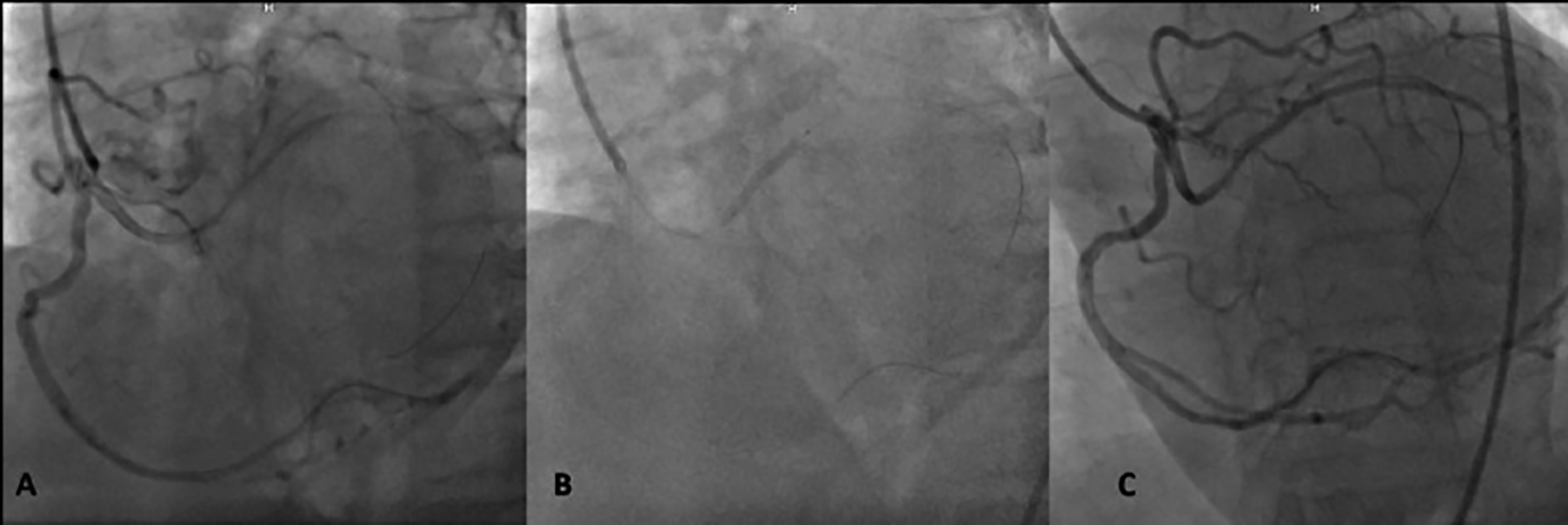 Figure 2: (A) Advancement of a guidewire to the anomalous artery was only possible following slight clockwise rotation of the Amplatz right 2 guiding catheter, which was stabilised with a guidewire inside the right coronary artery. (B) A drug-eluting stent was implanted. (C) Final angiographic view.
Figure 2: (A) Advancement of a guidewire to the anomalous artery was only possible following slight clockwise rotation of the Amplatz right 2 guiding catheter, which was stabilised with a guidewire inside the right coronary artery. (B) A drug-eluting stent was implanted. (C) Final angiographic view.
DISCUSSION
CAAs represent a wide spectrum of congenital cardiac anomalies ranging from benign conditions, such as separate LAD and LCx ostea, to conditions such as coronary artery originating from the pulmonary artery, which is infamously associated with an increased SCD risk. SCA with LMCA arising from the RCA is a rare form of CAA,2 classified as group II, according to Lipton’s classification.3 The mechanism of SCD and myocardial ischemia in CAA remain a controversial subject, as different mechanisms have been proposed by different investigators, including the slit-like ostium and tangential offset (in cases of LMCA originating from the right aortic sinus), artery compression between the dilated pulmonary artery and aorta during exercise, hypoplasia, and intussusception of the intramural segment of the anomalous artery.4 The rarity of the condition, along with its asymptomatic nature, has led to a paucity of data regarding the natural course and actual risk of SCD.
No randomised control studies or formal guidelines have addressed the optimal management of SCA. Reports describing both medical and surgical/interventional management approaches have been previously published.2 In asymptomatic patients, surgical/interventional management is generally reserved when ischemia is documented and/or concomitant atherosclerotic coronary artery disease is present.
In our case, the coronary anatomy proved to be a challenge for PCI. The inability to advance a guidewire across the culprit lesion using various guiding catheters, including JR, AL, Shepard’s crook and finally AR, led us to an unconventional approach. In this approach, the manoeuvrability and stability of the AR catheter were enhanced, using a guidewire that was advanced into the RCA. Few other reports have confirmed the observation that anomalous anatomy leads to difficulty in cannulation and catheter stability during PCI.5,6 No further intervention was planned, in the context of SCA management as the patient was asymptomatic and the anomaly itself was not associated with ischemia.
The main limitation of our report is the absence of CT coronary angiography data. However, as the patient was asymptomatic and presented no evidence of ischemia following revascularisation of the atherosclerotic lesion, CT imaging would not have altered the management plan and, therefore, was not performed.
In conclusion, RII SCA is usually an incidentally detected and rare condition. Medical management of asymptomatic patients with no evidence of myocardial ischemia is generally appropriate. Indications for interventional/surgical management include documented ischemia due to the course of the anomalous artery, or concomitant atherosclerotic disease, as seen in our patient. When PCI is attempted, a difficult cannulation of the anomalous artery should be anticipated, requiring wiring the main artery for catheter stabilisation and manipulation.
PATIENT’S CONSENT:
Written consent was obtained from the patient.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
KE: Conceptualisation, writing the original draft.
EB: Writing original draft.
NO: Writing, review and editing.
ET: Supervision.
REFERENCES
- Cheezum MK, Liberthson RR, Shah NR, Villines TC, O’Gara PT, Landzberg MJ, et al. Anomalous aortic origin of a coronary artery from the inappropriate sinus of valsalva. J Am Coll Cardiol 2017; 69(12):1592-608. doi: 10.1016/j. jacc.2017.01.031.
- Desmet W, Vanhaecke J, Vrolix M, Van de Werf F, Piessens J, Willems J, et al. Isolated single coronary artery: A review of 50,000 consecutive coronary angiographies. Eur Heart J 1992; 13(12): 1637-40. doi: 10.1093/oxfordjournals. eurheartj.a060117.
- Lipton MJ, Barry WH, Obrez I, Silverman JF, Wexler L. Isolated single coronary artery: Diagnosis, angiographic classification, and clinical significance. Radiology 1979; 130(1):39-947. doi: 10.1148/130.1.39.
- Angelini P, Walmsley RP, Libreros A, Ott DA. Symptomatic anomalous origination of the left coronary artery from the opposite sinus of valsalva. Clinical presentations, diagnosis, and surgical repair. Texas Hear Inst J 2006; 33(2):171-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/ 16878619.
- Hong LF, Luo SH, Li JJ. Percutaneous coronary intervention with anomalous origin of right coronary artery: Case reports and literature review. J Geriatr Cardiol 2013; 10(2):205-9. doi: 10.3969/j.issn.1671-5411.2013.02.011.
- Kuno T, Numasawa Y, Takahsashi T. Percutaneous Coronary Intervention for the Anomalous Left Coronary artery originating from the noncoronary cusp. Case Rep Cardiol 2016; 2016:1-4. doi: 10.1155/2016/2097174.