Non-operative Treatment of Gastric Fistula after Splenectomy Combined with Pericardial Devascularisation in Portal Hypertension
By Jun-min Zhu, Gang Chen, Zuo-yi JiaoAffiliations
doi: 10.29271/jcpsp.2021.03.334ABSTRACT
Portal hypertension may gradually lead to esophagogastric varices and splenomegaly in the decompensated stage of liver cirrhosis, which requires surgical treatment, if the disease worsens. Splenectomy with pericardial devascularisation is the routine surgery. One complex complication after splenectomy is upper gastric fistula which can seriously affect the perioperative recovery. Here, we present a case of a 51-year female patient, who recovered completely from non-operative treatment for gastric fistula after splenectomy and pericardial devascularisation. The occurrence of gastric fistula may be strongly related to preoperative preparation, intra-operative procedure and postoperative management. Therefore, personalised management is important for avoiding gastric fistula.
Key Words: Gastric fistula, Splenectomy, Pericardial devascularisation, Portal hypertension.
INTRODUCTION
Portal hypertension is a clinical syndrome. When the patients suffer from esophagogastric varices, splenomegaly, and hypersplenism, they need surgery for which the routine method is splenectomy combined with pericardial devascularisation.1 However, there are risks of complications following surgery, including gastric fistula, postoperative hemorrhage, etc.2 The incidence of gastric fistula is very low in recent years. But once it occurs, it is difficult to handle, and the treatment cycle is lengthy. Therefore, it is necessary to actively prevent gastric fistula in surgical practice.
CASE REPORT
A 51-year female patient presented with left upper abdominal pain with fatigue and anorexia for more than two months. After medical history taking, we found that she had no previous history of liver disease. Laboratory tests showed obvious abnormalities (Table I). Gastroscopy showed severe esophageal varices with positive red sign (Figure 1).
Computed tomography (CT) showed decompensated liver cirrhosis, portal hypertension, splenomegaly and pelvic fluid (Figure 1). She was diagnosed with decompensated liver cirrhosis, portal hypertension, splenomegaly, hypersplenism, and esophageal varices. The livers functions were evaluated as Child-Pugh A grade, and she underwent splenectomy, alongwith pericardial devascularisation. Postoperative pathological examination showed congestive splenomegaly and nodular cirrhosis (Figure 1).
On the 7th day after operation, stomach contents started draining from the spleen drainage tube. The patient complained of upper abdominal pain, fatigue and poor appetite, and white blood cell (WBC) count was 20.30 × 109/L. Abdominal CT showed a small amount of fluid around liver and splenic bed (Figure 1). Combined with the intraoperative findings, the diagnosis of postoperative gastric fistula was confirmed, and non-surgical treatment was designed for the patient. The patient was stopped oral intake; and a nasogastric tube and a nasojejunal feeding tube were placed under CT guidance. Antibiotics proton pump inhibitors and water-electrolyte balance were administered. In addition, her body temperature and the volume, color and nature of the drainage fluid were closely monitored, the drainage was recorded carefully (Table II). After 9 days of treatment, intravenous nutrition was stopped, and enteral nutrition was completely dependent on the jejunal feeding tube. As the status of the patient was stable, she was discharged with a jejunal feeding tube and an abdominal drainage tube. Forty days after operation, the drainage volume was about 10 ml/24h. After a few more days, the patient fully recovered and all tubes were removed.
Table I: Perioperative laboratory examinations. 2017.11.11, patient come to the hospital for the first time; 2017.11.21, after conservative treatment the patient was discharged; 2017.12.27 the patient was readmitted for surgical treatment; 2018.01.02, examinations on the day before the surgery; 2018.01.09, examinations on the day gastric fistula happened; 2018.01.16, examinations before the patient was discharged with a nutrition tube and an abdominal drainage tube.
|
Date |
WBC (×109/L) |
PLT (×109/L) |
TBIL (µmol/L) |
DBIL (µmol/L) |
IBIL (µmol/L) |
ALB (g/L) |
γ-GGT (U/L) |
ALP (u/L) |
PT(S) |
|
2017.11.11 |
4.39 |
23 |
29.6 |
14.9 |
14.7 |
36.7 |
140 |
205 |
15.1 |
|
2017.11.21 |
4.50 |
49 |
36.9 |
19.4 |
17.5 |
35.7 |
121 |
173 |
15.3 |
|
2017.12.27 |
2.30 |
24 |
39.8 |
11.2 |
28.6 |
37.7 |
127 |
150 |
12.5 |
|
2018.01.02 |
5.20 |
27 |
43.6 |
15.2 |
28.4 |
37.9 |
204 |
197 |
14.3 |
|
2018.01.09 |
20.30 |
106 |
33.8 |
25.7 |
8.1 |
31.2 |
124 |
159 |
- |
|
2018.01.16 |
7.80 |
176 |
55.4 |
34.8 |
20.6 |
36.4 |
147 |
198 |
- |
Table II: Record of drainage. It is reasonable to have a moderate amount of hemorrhagic fluid 1-2 days after surgery. On the 7th day afte surgery, gastric fistula was formed, so the drainage fluid increased, it was gray in colour, with food particles inside. With the fistula recovered, drainage fluid decreased (day 10-40).
|
Postoperative days (d) |
Volume (ml) |
Colour |
Contents |
Events |
|
1-2 |
250-350 |
light red |
a small amount of blood |
- |
|
3-6 |
50-150 |
faint yellow |
floc |
oral feeding |
|
7 |
1200 |
gray |
food |
gastric fistula formation |
|
8 |
1015 |
gray |
food |
- |
|
9 |
710 |
colorless |
floc |
- |
|
10-12 |
500-600 |
colorless |
floc |
|
|
13-15 |
400-500 |
colorless |
nothing |
removed stomach tube, discharged |
|
16-20 |
250-350 |
colorless |
nothing |
- |
|
21-25 |
80-120 |
colorless |
nothing |
- |
|
26-30 |
30-50 |
colorless |
nothing |
- |
|
31-35 |
20-30 |
colorless |
nothing |
oral feeding |
|
36-37 |
10-20 |
colorless |
nothing |
removed nutrition tube |
|
38-40 |
8-11 |
colorless |
nothing |
removed drainage tube |
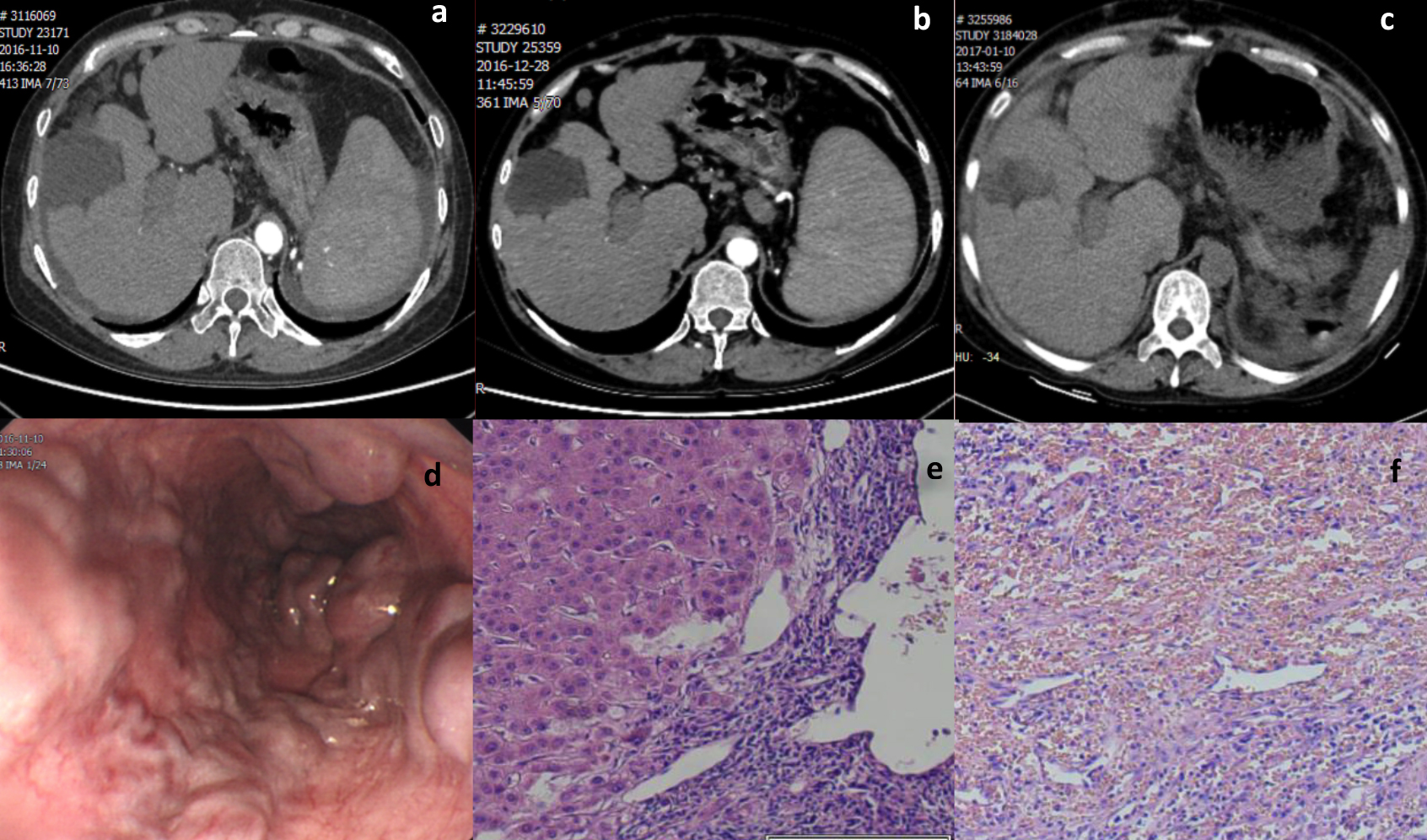 Figure 1: Perioperative figures of the patient. (a.b) decompensated liver cirrhosis, portal hypertension with collateral circulation formation, splenomegaly and a small amount of pelvic fluid; (c) small amount of fluid around the liver and spleen; (d) severe esophageal varices with red sign positive; (e. f) Postoperative pathological examination showed congestive splenomegaly and nodular cirrhosis.
Figure 1: Perioperative figures of the patient. (a.b) decompensated liver cirrhosis, portal hypertension with collateral circulation formation, splenomegaly and a small amount of pelvic fluid; (c) small amount of fluid around the liver and spleen; (d) severe esophageal varices with red sign positive; (e. f) Postoperative pathological examination showed congestive splenomegaly and nodular cirrhosis.
DISCUSSION
Timely detection and diagnosis is the key to the treatment of gastric fistula. Fever, epigastric pain, peritoneal irritation and intractable hiccups are the common symptoms of gastric fistula. Changes in the drainage fluid are often the initial manfestation.3 In order to obtain objective evidence, X-ray, upper gastrointestinal angiography, color ultrasound, CT and routine blood tests and drainage fluid amylase measurement should be conducted.4
Active and comprehensive treatments are crucial for gastric fistula. Non-surgical treatment includes multiple aspects. Firstly, no diet or water and enough gastrointestinal decompression are instituted. These prevent large amount of gastric juice and food from entering the abdominal cavity. Secondly, enteral nutrition by jejunal feeding tube can provide adequate nutrition and maintain gastrointestinal function. Thirdly, intravenous nutrition support, proton pump inhibition, somatostatin as well as antibiotics are also very important.5 During early period, total intravenous nutrition and electrolyte balance should be instituted.6 Besides, fluent drainage is the key to prevent infection-related complications. It is very helpful to make a detailed serial record of the characteristics of drainage fluid for the observation of treatment effect. Gastrointestinal fistulas may lead to subphrenic abscess, intestinal abscess, abdominal bleeding, pancreatic fistula, intestinal obstruction, sepsis and septic shock, eventually causing death.7 If the drainage tube placed during surgery is removed, ultrasound or CT-guided abdominal puncture is an option. Double lumen tube is better as it can douche while draining the fluid.
Intraoperative injury and postoperative ischemia-induced necrosis of the gastric wall are the two most important causes of gastric fistula.3 For patients with liver function of Child-Pugh grade B or below, surgery should be postponed until liver function returns to grade A.8 Mostly, in order to pursue complete devascularisation, the surgeons dissect the gastric wall too deeply and damage the muscle layer. Therefore, fistula is likely to occur due to mechanical damage and the resulting lack of blood supply. So, it is necessary to minimise the wound during surgery and properly repair the injury, and cover it with serosa. If the blood supply is poor, partial gastrectomy should be considered.
Some non-mechanical injuries may also increase the incidence of gastric fistula. Poor gastrointestinal motility or even stomach cramps will increase the pressure in the gastrointestinal cavity. Severe infections can impair the health condition; and the extravasated pancreatic juice from pancreatic fistula can cause caustic damage to the surrounding tissues, especially fresh wounds without complete serosal tissue protection. All the above factors can increase the incidence of gastric fistula or postoperative delayed bleeding.
In summary, early diagnosis and prompt conservative management can lead to successful repair of gastric fistula without the need of surgical treatment.
PATIENT’S CONSENT:
Informed consent was obtained from the patient.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
JZ, GC, ZJ: Conceived and designed the work. Acquisition, analysis of the data, drafted and revised the work, final approval of the version to be published, agreed to be accountable for all aspects of the work.
REFERENCES
- Ni YB, Gao PJ, Wang D, Li Z, Zhu JY. Esophagogastric devascularization without splenectomy in portal hypertension: Safe and effective? Hepatobiliary Pancreat Dis Int 2015; 14(3): 276-80. doi: 10.1016/s1499-3872(15) 60328-9.
- Chen H, Yang F, Li TT, Zhang KN, Sun ZG, Yu CZ, et al. Comparison of efficacy of laparoscopic and open splenectomy combined with selective and nonselective pericardial devascularization in portal hypertension patients. Surg Laparosc Endosc Percutan Tech 2018; 28(6):401-403. doi: 10.1097/ SLE.0000000000 000581.
- Harrison BJ, Glanges E, Sparkman RS. Gastric fistula following spenectomy: Its cause and prevention. Ann Surg 1977; 185(2):210-3. doi: 10.1097/00000658-197702000-00013.
- Taniguchi Y, Kurokawa Y, Mikami J, Tanaka K, Miyazaki Y, Makino T, et al. Amylase concentration in drainage fluid as a predictive factor for severe postoperative pancreatic fistula in patients with gastric cancer. Surg Today 2017; 47(11): 1378-83. doi: 10.1007/s00595-017-1521-y.
- Schlager A, Arps K, Siddharthan R, Rajdev P, Heiss KF. The "omega" jejunostomy tube: A preferred alternative for postpyloric feeding access. J Pediatr Surg 2016; 51(2):260-3. doi: 10.1016/j.jpedsurg.2015.10.073.
- Gómez-Hoyos E, Buigues AO, Ballesteros Pomar MD, Casariego AV, Delgado YG, Ocón Bretón MJ, et al. Development of hyponatremia in non-critical patients receiving total parenteral nutrition: A prospective, multicenter study. Clin Nut 2019; 38(6):2639-44. doi: 10.1016/j.clnu. 2018.11.014.
- Persson S, Rouvelas I, Irino T, Lundell L. Outcomes following the main treatment options in patients with a leaking esophagus: A systematic literature review. Dis Esophagus 2017; 30(12):1-10. doi: 10.1093/dote/dox108.
- Bleszynski MS, Bressan AK, Joos E, Morad Hameed S, Ball CG. Acute care and emergency general surgery in patients with chronic liver disease: How can we optimize perioperative care? A review of the literature. World J Emerg Surg 2018; 13: 32. doi: 10.1186/s13017-018 -0194-1.