Mechanical Prosthetic Aortic Valve Thrombosis Treated with Low-dose Ultra Slow Infusion of Tissue-type Plasminogen Activator during Pregnancy
By Ulku Mete Ural1, Aybike Uysal1, Emrah Erdal2Affiliations
doi: 10.29271/jcpsp.2021.03.369Sir,
Prosthetic valve thrombosis (PVT) is extremely rare in patients with aortic valve replacement and can be a life-threatening condition for both the mother and fetus, if it develops in pregnant women; and it requires immediate treatment. The treatment options during pregnancy include valve replacement, surgical thrombectomy, and thrombolytic therapy.1 The surgical approach in pregnancy is associated with elevated maternal and fetal mortality.2
A 21-year pregnant woman, who had a history of ventricular septal defect operation at age 10 years and mechanical aortic valve replacement at 11 years, presented with prosthetic valve thrombosis at 30 weeks of gestation, under the treatment of anticoagulation with 6000 IU subcutaneous enoxaparin® twice a day. Critical aortic valve stenosis was detected and transthoracic echocardiography (TTE) indicated an elevated mean transaortic pressure gradient of 60 mmHg and transesophageal echocardiogram (TEE) demonstrated thrombosis bigger than 12 millimeters (Figure 1). Tissue-type plasminogen activator (t-PA) treatment was started with a low-dose (25 mg) and ultra slow infusion (25 hours). After the first dose, repeat TTE showed a decrease in mean transaortic pressure gradient, but residual thrombus with a diameter of 10-12 mm was present. Second thrombolytic dose was repeated 24 hours later in the same way (low-dose, ultra slow infusion). After the second dose, repeat TTE showed 5-6 mm thrombus and decreased mean aortic gradient of 16 mmHg (Figure 2). The treatment was considered successful because of >75% reduction in thrombus size. Subsequently, warfarin and heparin treatment was started. When international normalised ratio (INR) value exceeded 2.5, heparin was stopped and warfarin was continued till delivery.
At 38th gestational week, she delivered a 3 kg healthy male infant with a 7-9 Apgar score, by caesarian section under general anesthesia. The patient and her baby were discharged on postpartum day 6 without any complications.
Tissue-type plasminogen activator (t-PA) is a highly fibrin- specific agent without any systemic thrombolytic effects. t-PA passes the placenta at a very low rate; but carries the risks of systemic embolization, subchorionic bleeding, ablatio placenta, uteroplacental insufficiency, fetal demise, and bleeding.3
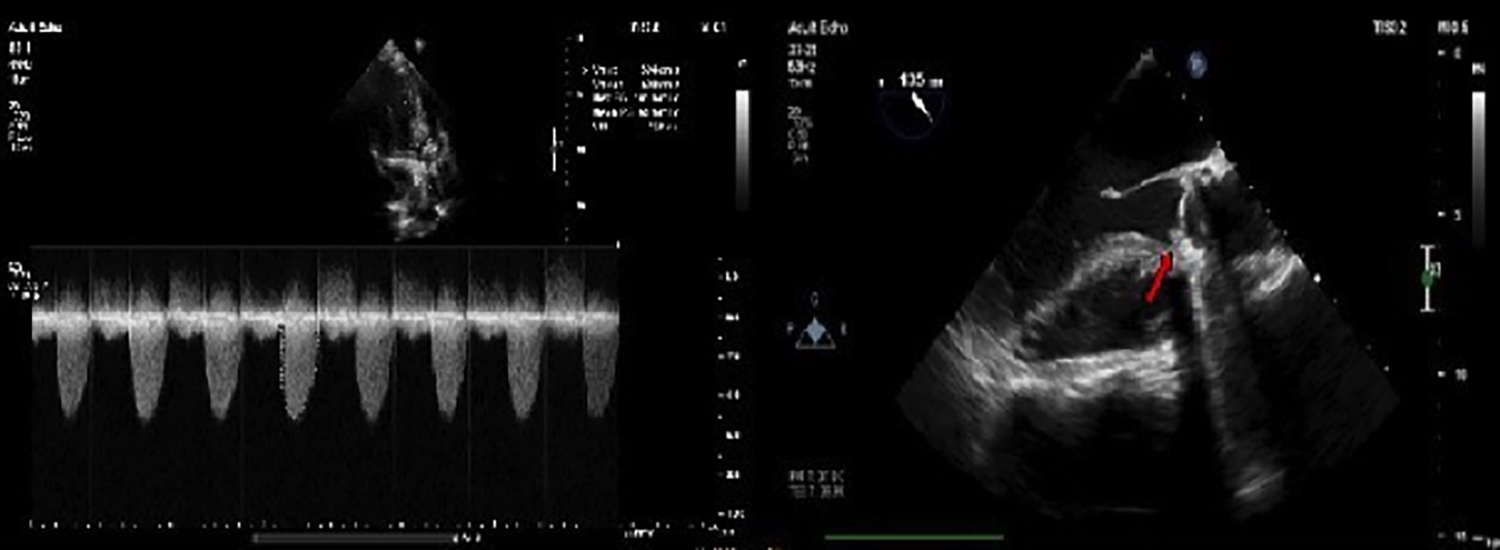 Figure 1: Critical aortic stenosis, elevated mean transaortic pressure gradient of 60 mmHg and thrombosis bigger than 10 millimeter.
Figure 1: Critical aortic stenosis, elevated mean transaortic pressure gradient of 60 mmHg and thrombosis bigger than 10 millimeter.
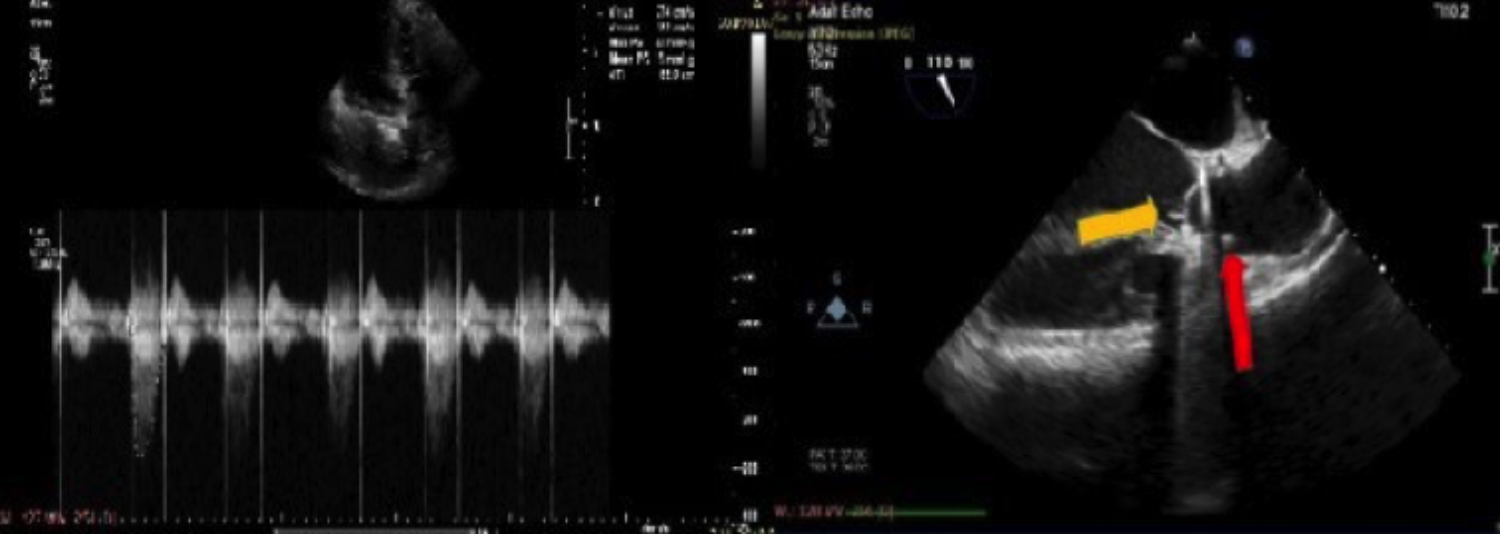 Figure 2: Decreased mean aortic gradient of 16 mmHg and 5-6 mm thrombus after the second thrombolytic treatment.
Figure 2: Decreased mean aortic gradient of 16 mmHg and 5-6 mm thrombus after the second thrombolytic treatment.
There is no consensus about the optimal treatment modality, or the type, dose, and route of administration of thrombolytic drugs. A large, prospective study which included 182 non-pregnant patients with 220 episodes of PVT treated with low-dose slow infusion (6 hours) of t-PA regimen, demonstrated a thrombolytic success of 86% and a complication rate of 10.5% without mortality.4 In another trial, which involved 114 pregnant and non-pregnant patients with 120 episodes, who were treated with low-dose ultra slow (25 hours) infusion of t-PA protocol, the outcome was associated with 90% thrombolytic success and 6.7% complication rate.5 Further prolongation of the infusion time may be significant in reducing complication rates without reducing treatment efficacy. The ultra slow protocol seems to be safer than slow protocol in terms of complication rates (6.7% vs. 10.5%, respectively). The overall thrombolytic success rate in ultra slow infusion is higher than the slow infusion strategy (90% vs. 86%).
Low-dose, ultra slow infusion of t-PA seems to be an effective treatment modality with high success and low complication rates; and may be considered as first-line therapy in pregnant women with prosthetic valve thrombosis.
CONFLICT OF INTEREST:
Authors declared that there is no conflict of interest for this article.
AUTHORS’ CONTRIBUTION:
UMU, AU, EE: Design, data collection, writing and critical review.
REFERENCES
- Van Hagen IM, Roos-Hesselink JW, Ruys TP, Merz WM, Goland S, Gabriel H, et al. ROPAC Investigators and the EURObservational Research Programme (EORP) Team. Pregnancy in women with a mechanical heart valve: Data of the European Society of Cardiology Registry of Pregnancy and Cardiac Disease (ROPAC). Circulation 2015; 132(2):132-42. doi: 10.1161/Cırculatıonaha.115.015242.
- Weiss BM, Von Segesser LK, Alon E, Seifert B, Turina MI. Outcome of cardiovascular surgery and pregnancy: A systematic review of the period 1984-1996. Am J Obstet Gynecol 1998; 179(6 Pt 1):1643-53. doi: 10.1016/s0002- 9378(98)70039-0.
- Özkan M, Çakal B, Karakoyun S, Gürsoy OM, Çevik C, Kalçık M, et al. Thrombolytic therapy for the treatment of prosthetic heart valve thrombosis in pregnancy with low-dose, slow infusion of tissue-type plasminogen activator. Circulation 2013; 128(5):532-40. doi: 10.1161/ Cırculatıonaha.113.001145.
- Özkan M, Gündüz S, Biteker M, Astarcioglu MA, Çevik C, Kaynak E, et al. Comparison of different TEE-guided thrombolytic regimens for prosthetic valve thrombosis: the TROIA trial. JACC Cardiovasc Imaging 2013; 6(2):206-16. doi: 10.1016/j.jcmg.2012.10.016.
- Özkan M, Gündüz S, Gürsoy OM, Karakoyun S, Astarcıoğlu MA, Kalçık M, et al. Ultraslow thrombolytic therapy: A novel strategy in the management of PROsthetic MEchanical valve Thrombosis and the prEdictors of outcomE: The Ultra-slow PROMETEE trial. Am Heart J 2015; 170(2):409-18. doi: 10.1016/j.ahj.2015.04.025.