Late Diagnosis of Paracetamol Poisoning is Always Lethal in Young Adult
By Sehrish Naz, Safia Fatima, Muhammad AamirAffiliations
doi: 10.29271/jcpsp.2020.06.655ABSTRACT
Acetaminophen has a remarkable safety profile when prescribed in proper therapeutic doses, but hepatotoxicity can occur when misused or after an overdose. The principal toxic metabolite of acetaminophen is N-acetyl-p-benzoquinone imine (NAPQI). Toxicity should be considered in all suspicious cases because of the ubiquitous and initially asymptomatic nature of acetaminophen intoxication. A case of 29-year male soldier is discussed, who presented with pain in abdomen, vomiting and jaundice of sudden onset. The diagnosis of ischemic liver damage was made at initial presentation. Raised liver function tests and elevated prothrombin time were the first indication to this condition, which were proven by detection of acetaminophen in blood and urine by liquid chromatography-mass spectrometry. Further supportive evidence of hepatic necrosis was provided by an ultrasound abdomen, giving the final diagnosis of acetaminophen poisoning causing drug-induced liver injury.
Key Words: Acetaminophen poisoning, Drug-induced liver injury, Fulminant hepatic failure, N-acetylcysteine, N-acetyl-p-benzoquinone imine (NAPQI).
INTRODUCTION
According to USA documented data, acetaminophen or N-acetyl-para-aminophenol (APAP), commonly sold as paracetamol, is the most widely used analgesic and antipyretic. The dose of up to 4000 mg per 24 hours of acetaminophen is safe as advertised by the US Food & Drug Administration (FDA) and does not produce toxic effects. If the dose exceeds above 150 mg/kg as a single dose, toxic effects are produced resulting in liver failure. About 90 percent of the APAP is channeled into phase II metabolic pathway and converted to glucouronidated and sulfated metabolites, which are eliminated from the body in the urine.1 Almost 10 percent of the APAP is shunted by cytochrome (CYP) 2E1 to phase I oxidation, resulting in formation of a highly reactive toxic metabolite, known as, N-acetylpara-benzo-quinone imine (NAPQI).2 A very small amount, i.e., almost two percent of APAP is excreted unchanged in the urine. Hepatotoxicity induced by acetaminophen overdose occurs through production of the harmful NAPQI metabolite. When present in excessive quantities, it results in depletion of glutathione, oxidative stress and mitochondrial dysfunction leading to depletion in adenosine triphosphate (ATP) stores.
Correct initial diagnosis of paracetamol overdose is critical. Timely identification and early therapy may prevent significant morbidity and mortality.
Four established sequential stages of paracetamol-induced hepatotoxicity should be considered upon presentation to clinic (Table I).
Table I: Stages of paracetamol-induced hepatotoxicity.
|
Stage |
Time post ingestion |
Description |
|
Recovery phase |
First 24 hours |
Nonspecific symptoms like anorexia, nausea, vomiting. |
|
24 - 72 hours |
Right upper quadrant abdominal pain (common). In severe poisoning, AST, ALT bilirubin and PT (usually reported as the INR) elevated. Persistant Vomiting with symptoms of liver failure. |
|
|
72 – 96 hours |
Peaking of serum ALT, AST, bilirubin, and INR. |
|
|
After 96 hours |
Resolution of hepatotoxicity or progression to multiple organ failure. |
Rumack-Matthew nomogram is a widely acceptable tool used in the management of acetaminophen overdose.3 It is applicable only in the event of single acute ingestion or if the acuity of ingestion is known to be within 24 hours. Acetaminophen-induced liver injury is known to raise serum aminotransferases above 10,000 IU/L.4 New biomarkers available are acetaminophen / cysteine protein adducts which indicate exposure to acetaminophen.5
Table II: Important laboratory parameters of this patient.
|
Parameters |
Day 1 of referral |
Day 2 |
Day 3 |
Day 4 |
|
1.Total bilirubin μmol/l (0-17) |
414 |
606 |
686 |
938 |
|
2.Serum ALT U/l (upto 42) |
7046 |
1843 |
2591 |
279 |
|
3. Serum ALP |
148 |
152 |
180 |
113 |
|
4. serum AST U/l (˂37 ) |
Not done |
5035 |
2361 |
Not done |
|
5. Prothrombin time (0-14 sec) |
16 |
18 |
20 |
20 |
|
6. Hemoglobin level g/dL |
10.5 |
Not done |
9.8 |
10.5 |
|
7.Serum Gamma GT U/l (upto 50 U/l) |
72 |
Not done |
Not done |
53 |
|
8.Total leucocyte count (4-10 ˟ 10⁹/L) |
17.4 |
Not done |
37.9 |
63.2 |
|
9. serum urea mmol/l (2.1- 4.7) |
Within normal reference limits |
4.2 |
9.9 |
9.2 |
|
10. serum creatinine mmol/l (80-115) |
Within normal reference limits |
109 |
398 |
304 |
|
11. Other
|
Hepatitis B (HBsAg): Negative |
serum LDH: 9928 |
PTTK: 54 (0-32 sec) |
PTTK: 56 (0-32 sec) |
|
Acetaminophen level (blood): 9874 ng/ml |
Acetaminophen level (blood): 6358 ng/ml |
Acetaminophen level (blood): 1544 ng/ml |
Acetaminophen level (blood): Undetectable |
|
|
Hepatitis C (Anti HCV): Negative |
Acetaminophen level (urine): Positive |
Serum K: 5.6 mmol/l |
Nasobronchial lavage for C/S: Negative |
|
|
Serum ferritin: |
Acetaminophen level (gastric lavage): Negative |
Serum CK: 3135 |
IgG Antibody to mycoplasma pneumoniae: Negative |
|
|
CMV–DNA PCR (Quant): Negative |
Liver kidney microsomal antibodies: Negative |
Serum CK-MB: 187 |
IgM Antibody to mycoplasma pneumoniae: Negative |
|
|
|
Alpha fetoprotein: 4.7 ng/ml (<8.5) |
Fluid for C/S: negative |
|
Treatment includes oral or intravenous N-acetylcysteine, a glutathione precursor that inactivates and converts NAPQI to harmless metabolites.6,7 Overall, timely identification and prompt treatment of acetaminophen overdose is critical because it can lead to significant morbidity and mortality, if untreated.
CASE REPORT
A 29-year male soldier was referred from Central Military Hospital, Mardan, on 30th March 2019 after 5 days of hospital stay with a provisional diagnosis of ischemic liver damage. He was immediately admitted in Pak Emirates Military Hospital (PEMH), Rawalpindi, and transferred to Armed Forces Liver Transplant Unit.
Patient was in the usual state of health six days back when he suddenly developed pain in abdomen localized to the right upper quadrant along with recurrent vomiting and diaphoresis. Later on, he developed jaundice. Despite continued deterioration, no suspicion of paracetamol poisoning was made. A detailed general physical examination was carried out, which revealed bilateral scleral icterus and diffuse jaundice. Upon abdominal examination, marked tenderness over right upper quadrant was observed, while no abnormality was detected on his cardiopulmonary examination. His significant laboratory findings during his hospital stay at PEMH are shown above in the Table II.
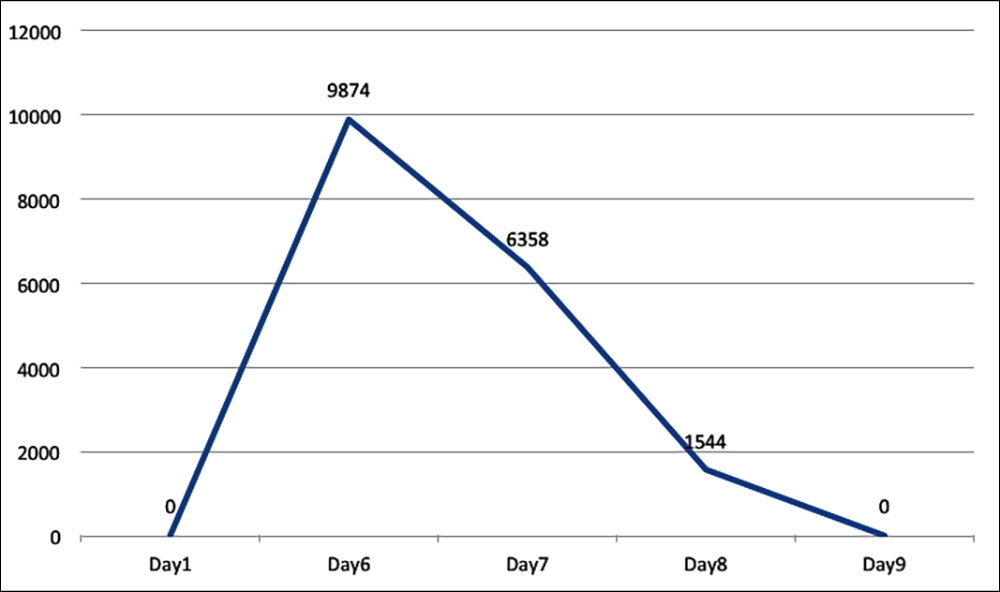 Figure 1: Graph constructed by the patient’s readings of aceta-minophen levels (blood) taken at day 6, 7 and 8 of acetaminophen overdose.
Figure 1: Graph constructed by the patient’s readings of aceta-minophen levels (blood) taken at day 6, 7 and 8 of acetaminophen overdose.
By day 2 of referral, patient went into acute fulminant hepatitis but no specific cause was established. The manag-ing team sent the blood to Armed Forces Institute of Pathology (AFIP), Rawalpindi, for paracetamol levels. Acetaminophen (blood) levels were found to be 6358 ng/ml. The patient deteriorated with rapid rise in serum total bilirubin.
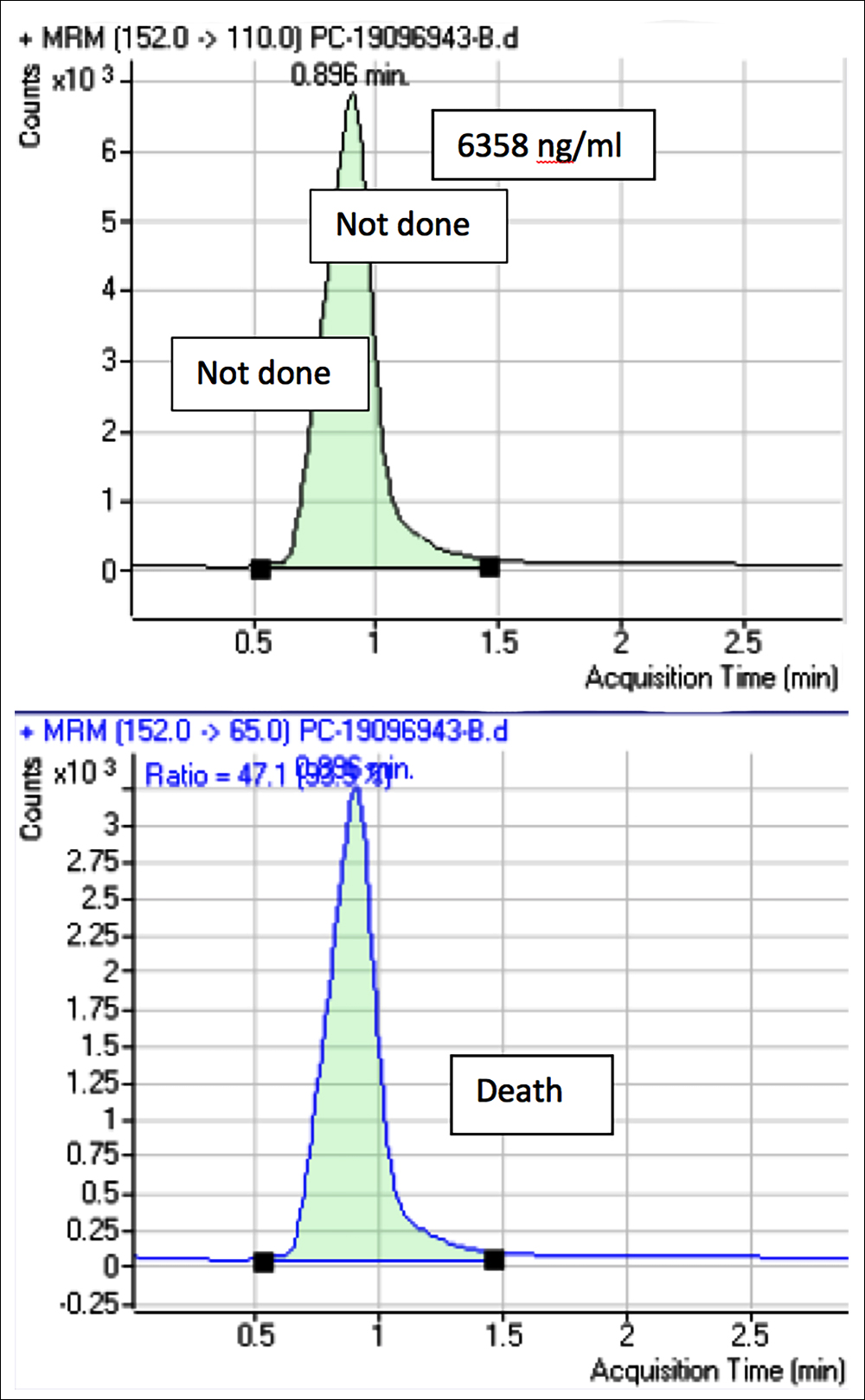 Figure: 2 Liquid chromatography-mass spectrometry chromatogram of this patient, showing significant peak of acetaminophen when analysed.
Figure: 2 Liquid chromatography-mass spectrometry chromatogram of this patient, showing significant peak of acetaminophen when analysed.
By the third day of referral, despite intensive care and appropriate medication, the patient progressed to multisystem organ failure with poor prognosis and developed ascites with neurological symptoms. Conservative treatment was recommended. On day four of referral, the liver transplant team marked him as the poor candidate for liver transplant due to poor prognosis and multiple organ system failure, so conservative treatment was continued. Registrar toxicology department retrieved the patient’s past sample taken at the admission from the PEMH Hospital, Rawalpindi and performed acetaminophen level (blood), which was found to be 9874 ng/ml, i.e., greater than the levels observed on the 2nd day of referral.
Based on above mentioned findings, this case was labelled as drug-induced liver injury progressing to multi-system organ failure. Poor prognosis, complications and the course of illness of the disease were counselled to the family. Maximal pressor support was given and the patient was managed on the ventilator. Despite continued efforts of the medical team, he became unresponsive, continued to decline and expired.
DISCUSSION
Acetaminophen is the commonest over the counter (OTC) analgesic readily available in the market. According to the US data, it comprises 50% of cases of drug-induced liver injury. The patient discussed above is the classic case of acute acetaminophen poisoning, who presented with severe abdominal pain in the upper right quadrant, coagulopathy, diffuse jaundice and hepatomegaly along with biochemical evidence of significantly raised serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin levels and associated deranged renal function tests leading to multi-system organ failure. As the time of ingestion was not known in this case, the Rumack-Matthew nomogram for prediction of hepatic injury could not be applied. Based on the above mentioned clinical picture, biochemical findings and radiological evidence of hepatomegaly along with peri-portal and gall bladder wall edema, a diagnosis of drug-induced liver injury due to acetaminophen overdose was made.
Acetaminophen-protein adducts using high-performance liquid chromatography (HPLC) is a latest test available for assessing APAP-induced hepatotoxicity.8 Several other clinically important serum markers indicating mitochondrial damage including glutamate dehydrogenase (GDH), nuclear DNA (nDNA) and mtDNA are increasingly being used.9 Early molecular biomarker, miRNA-122 is also available for assessing hepatic injury. It notably increases even before the rise of serum ALT levels. These pertinent laboratory tests support collection of clinical findings for better prediction of patient’s prognosis, morbidity and mortality.10 It will broaden the horizon and will clear the queries regarding treatment protocol and decision making.
A multidisciplinary approach is vital in dealing with these cases. The treating panel should also include a psychiatrist as well. Detailed psychiatric assessment should be made mandatory, especially in patients who have intentionally overdosed on acetaminophen. Awareness programmes should be arranged to educate people to avoid its misuse. Advance therapeutic and clinical researches should be carried out for better understanding of molecular and sub-molecular pathways.11 It will help combat hepatic damage at very early stage. Molecular advancements in the field of health and medicine will prove as a game-changer to tackle this devastating yet curable event.
CONFLICT OF INTEREST:
The authors declared that they had no conflict of interests.
PATIENT’S CONSENT:
Consent could not be taken due to critical condition and then the death of the patient.
AUTHORS’ CONTRIBUTION:
All authors contributed to the study design, interpretation, drafting and preparation of the manuscript and have read and approved the final version. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
REFERENCES
- Borude P, Bhushan B, Chavan H, Weemhoff JL, Jaeschke H, Krishnamurthy P, et al. P53 regulates progression of injury and liver regeneration after acetaminophen overdose. FASEB J 2017; 31(1 Supplement):531.
- Burns MJ, Friedman SL, Larson A. Acetaminophen (paracetamol) poisoning in adults: Pathophysiology, presentation, and diagnosis. UpToDate Waltham, MA: UpToDate; 2017.
- Bateman DN. Acetaminophen (Paracetamol). Crit Care Toxicol 2016:1-25.
- Wong A, Graudins A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin Toxicol (Phila) 2017; 55(8): 879-92.
- Levine M, O’Connor AD, Padilla-Jones A, Gerkin RD. Comparison of prothrombin time and aspartate amino-transferase in predicting hepatotoxicity after acetamino-phen overdose. J Med Toxicol 2016; 12(2):218.
- Chiew AL, Gluud C, Brok J, Buckley NA. Interventions for paracetamol (acetaminophen) overdose. Cochrane Database Syst Rev 2018; 2:CD003328.
- Mahmoudi GA, Astaraki P, Mohtashami AZ, Ahadi M. N-acetylcysteine overdose after acetaminophen poisoning. Int Med Case Rep J 2015; 8:65-9.
- Thomas KC, Wilkins DG, Curry SC, Grey TC, Andrenyak DM, McGill LD, et al. Detection of acetaminophen-protein adducts in decedents with suspected opioid-aceta- minophen combination product overdose. J Forensic Sci 2016; 61(5):1301-6.
- Woolbright BL, Jaeschke H. Biomarkers of mitochondrial injury after acetaminophen overdose: glutamate dehydrogenase and beyond. In: Will Y, Dykens JA, Eds. Mitochondrial Dysfunction Caused by Drugs and Environmental Toxicants. John Wiley & Sons, Inc. 2018: p. 373-82.
- Irshad M, Malik M, Furqan A. Intravenous paracetamol in pediatrics: A global perspective. Anaesth Pain Intensive Care 2012; 16(3):311-4.
- Mukhtar I, Anwar H, Hussain G, Rasul A, Naqvi SA, Faisal MN, et al. Detection of Paracetamol as substrate of the gut microbiome. Pak J Pharm Sci 2019; 32 (Supplementary): 751-7.