Dehydroepiandrosterone Sulfate (DHEAS) Levels in Polycystic Ovarian Syndrome (PCOS)
By Sikandar Hayat Khan1, Syeda Arsheen Rizvi2, Rahat Shahid3, Robina Manzoor4Affiliations
doi: 10.29271/jcpsp.2021.03.253ABSTRACT
Objective: To compare DHEAS levels among subjects with and without PCOS, evaluating differences between lean-PCOS or obese-PCOS phenotype for insulin resistance, anthropometric indices, glycemic and lipid parameters.
Study Design: Descriptive study.
Study Place and Duration of Study: PNS Hafeez Naval Hospital, Islamabad, Pakistan, from January 2018 to August 2019.
Methodology: Three hundred and twenty-eight subjects were included in the study for evaluation. PCOS was defined as per Rotterdam criteria, while insulin resistance, anthropometric measurements, various hormonal and biochemical analyses were carried out as per standard protocols. Hirsutism was calculated as per modified Ferrimen Gallwey score and free androgen index (FAI) was calculated using formula as: FAI = [(Total testosterone/Sex hormone binding globulin (SHBG)] x100. These subjects underwent clinical biochemical evaluation and were segregated into 2 groups: lean-PCOS and obese-PCOS.
Results: DHEAS levels were higher in subjects with PCOS [(171.50) (111.75-244.25) ug/dl], n=164] than in subjects without PCOS [(130.50) (78.95-189.75) ug/dl, n=164, p<0.001]. Area under curve (AUC) in diagnosing PCOS was highest for modified FG score [0.802, p<0.001], followed by FAI [0.785, p<0.001]. Total testosterone [0.743, p<0.001] and DHEAS [0.637, p<0.001]. DHEAS levels were found to be inversely related to age, anthropometric indices, glycemia, dyslipidemia, nephropathy and reproductive hormones. The DHEAS in lean-PCOS was higher than obese female subjects with or without PCOS.
Conclusion: DHEAS levels were high in lean-PCOS in comparison to obese-PCOS and non-PCOS females. However, receiver operating curve (ROC) analysis showed DHEAS as a weaker marker for diagnosing PCOS than FAI and modified FG score.
Key Words: DHEAS, Polycystic ovarian syndrome (PCOS), Homeostasis model assessment for insulin resistance (HOMAIR), Rotterdam criteria, Free androgen index.
INTRODUCTION
Dehydroepiandrosterone sulfate (DHEAS) is considered a pre-hormone produced by the adrenal cortex and can be converted into DHEA. DHEA is considered as active hormone with multiple action including further conversion into dihydrotestosterone (DHT) and testosterone (Te). Literature search has identified various roles of DHEAS including action as a neurosteroid, but its role as week adrenal steroid is also well-established.1 Recent evidence points towards the fact that upto 20-30% of females with PCOS like characteristics have excessive secretion of adrenal androgens including DHEAS, thus highlighting the possibility that adrenal androgens could have a role in the pathogenesis of PCOS.2
This phenomena, if taken in exactness, thus clearly indicates multi-faceted picture of PCOS, needing further research. There remains a possibility that females with higher DHEAS could affect hypothalamic pituitary axis (HPA) and hypothalamic pituitary ovarian (HPO) axis; and thus related with cardiovascular diseases.
It was reported that female subjects with raised DHEAS are more associated with long-term cardiovascular disease risk as shown by higher fibrinogen levels, dyslipidemia and pro-inflammatory markers.3 Another significance. A Turkish study has shown that adrenal androgen are closely correlated with PCOS subjects not only in the patients themselves but also in their siblings highlighting the probability of inheritability among some subjects.4 An interventional animal model study demonstrated excessive adrenal androgen exposure during fetal life causes later onset PCOS type disease in Rhesus monkeys.5 The study was also able to demonstrate an association between insulin resistance and DHEAS levels.
The question arises whether these lean and obese PCOS differ in terms of hyperandrogenemia. An earlier study has observed higher DHEAS in PCOS subjects with androgen excess to have lower insulin resistance than PCOS patients with lower DHEAS. 6 Similarly, it was suggested that non-obese PCOS subjects having androgen excess along with raised DHEAS levels and low Luteinizing hormone (LH) concentrations than obese PCOS types indicating androgen levels to be associated with DHEAS.7 Contrary to that, another study has not shown good pregnancy outcomes in PCOS patients with low DHEAS levels once managed by dexamethasone.8 Based upon the contrasting evidence relating DHEAS and PCOS, the objective of this study was to compare DHEAS levels among subjects with and without PCOS, among lean PCOS and obese PCOS phenotypes and correlate DHEAS levels with insulin resistance, dyslipidemias, diabetic and inflammatory markers in the studied groups.
METHODOLOGY
The study was approved by Ethical Review Committee of PNS Hafeez Naval Hospital, Islamabad. It was a descriptive study conducted from January 2018 to August 2019. Female subjects in the reproductive age group having menstrual problems were the target population and were sent initially to Gynecology Outpatient Department for initial inclusion in the study. Initial referred patients were interviewed in Gynecology Department. Participants who had history of metabolic disorder, specially hypertension, heart disease, diabetes including prior gestational diabetes, autoimmune disorders, gynecological disorders not related with PCOS, having pregnancy or ongoing treatment for any skin disease were major exclusions. These participants were further considered for onward examination and referral for anthropometric evaluation and laboratory testing during their follicular phase of menstrual cycle. Patients who finally reported to Lab, as per the probable dates discussed by gynecologists, were formally offered for inclusion into study by signing a written signed consent after explanation about type of data collection and later usage for research purpose. Only volunteering subjects were finally included for anthropometric examination and laboratory and radiological evaluation.
Detailed history on study questionnaire was recorded and clinical examination was conducted including hirsutism assessment as per modified Ferriman-Gallwey score (mFG score) at the Gynecology Department.9 POCS was diagnosed as per Rotterdam criteria.10 Anthropometric parameters were assessed as per standard protocols. A diagnosis of PCOS was made by radiologist as per the Rotterdam criteria, once there was presence of >12 follicles (size ranging between 2-9 mm diameter) either with or without ovarian volume of < 10 ml.11
Finally, included subjects were 328. Approximately 10 ml blood was collected for measuring HbA1c, fasting glucose, lipids, total testosterone, sex hormone binding globulin (SHBG), albumin, DHEAS, and insulin. After Lab testing, patients were referred to for radiological examination. Random access clinical chemistry analyser (Selectra ProM) was utilised to measure fasting glucose by GPO-PAP method, total cholesterol by CHOD-PAP method, triglycerides by GOD-PAP method. LDL cholesterol and HDL cholesterol were analysed by direct enzymatic, selective end-point method, using selective detergent and accelerator selective detergent method on Selectra-proM clinical chemistry analyser. Glycated hemoglobin (HbA1c), luteinising hormone (LH), follicle stimulating hormone (FSH), total testosterone, and sex-hormone binding globulin (SHBG) was measured by Chemiluminescent Micro-particle Immuno Assay (CMIA) on ARCHITECT iSystem supplied by Abbot Diagnostics. Serum insulin was analysed by chemiluminescence method on Immulite® 1000. Insulin resistance was measured using homeostasis model assessment for insulin resistance (HOMAIR) as per the criteria of Mathew’s et al. PCOS cases were divided into two types: One type being obese-PCOS and other the lean-PCOS. Obesity was dealt both by using BMI with cut-offs of 25 kg//m2 and also according to WHtR by using the cut-off of 0.5.
All data were initially entered into Excel Microsoft programme and later transferred to SPSS-version 24. Continuous data were given as mean ± standard deviation (S.D) and median (IQR: Q1-Q3). The results of DHEAS and HOMAIR were not found to conform standard normality pattern, so DHEAS levels were evaluated and compared in subjects with and without PCOS by using non-parametric Mann-Whitney U-test. Furthermore, the components included in the criteria including ultrasound PCOS diagnosis, and oligo/anovulation were also evaluated by using independent sample t-test. Pearson’s correlation between DHEAS and insulin resistance was measured with age, anthropometric indices, biochemical and hormonal parameters. Later, area under curve (AUC) analysis for diagnosing PCOS for free androgen index (FAI), modified FG score, total testosterone and DHEAS were measured by using ROC method. Keeping in view the non-Gaussian distribution of DHEAS with BMI, PCOS presence or absence, and with insulin resistance data, lean and obese PCOS and non-PCOS subjects were analysed by Kruskal-Wallis test. SPSS graphics were utilised to visualise the differences within PCOS and non-PCOS groups for BMI-defined obesity. A p-value of <0.05 was considered as significant.
RESULTS
The average age among the subjects in this study was 27.91 (+7.64) years. DHEAS levels were higher in subjects with PCOS [(171.50(111.75-244.25) ug/dl], n=164] than in subjects without PCOS [(130.50(78.95-189.75) ug/dl, n=164, p<0.001] as shown in Figure 1. As per ultrasound PCOS, presence or absence (175.55 ± 92.29 ug/dl, n=84 vs. 161.20 ± 96.01 ug/dl, n=244), the DHEAS levels were not found to be significant between the two groups. (p=0.234).There was no significant difference among subjects with complaints of oligo/anovulation (171.64 + 99.64 ug/dl, n=202) or otherwise (154.03 + 86.73 ug/dl, n=126) for DHEAS levels (p = 0.103). Area under curve (AUC) in diagnosing Rotterdam formulated PCOS criteria was measured to be highest for modified FG score [0.802(95% CI: 0.753-0.850), p<0.001], followed by FAI [0.785(95% CI: 0.735-0.836), p<0.001], total testosterone [0.743(95% CI: 0.691-0.796), p<0.001] and DHEAS [0.637(95% CI: 0.578-0.697), p<0.001].
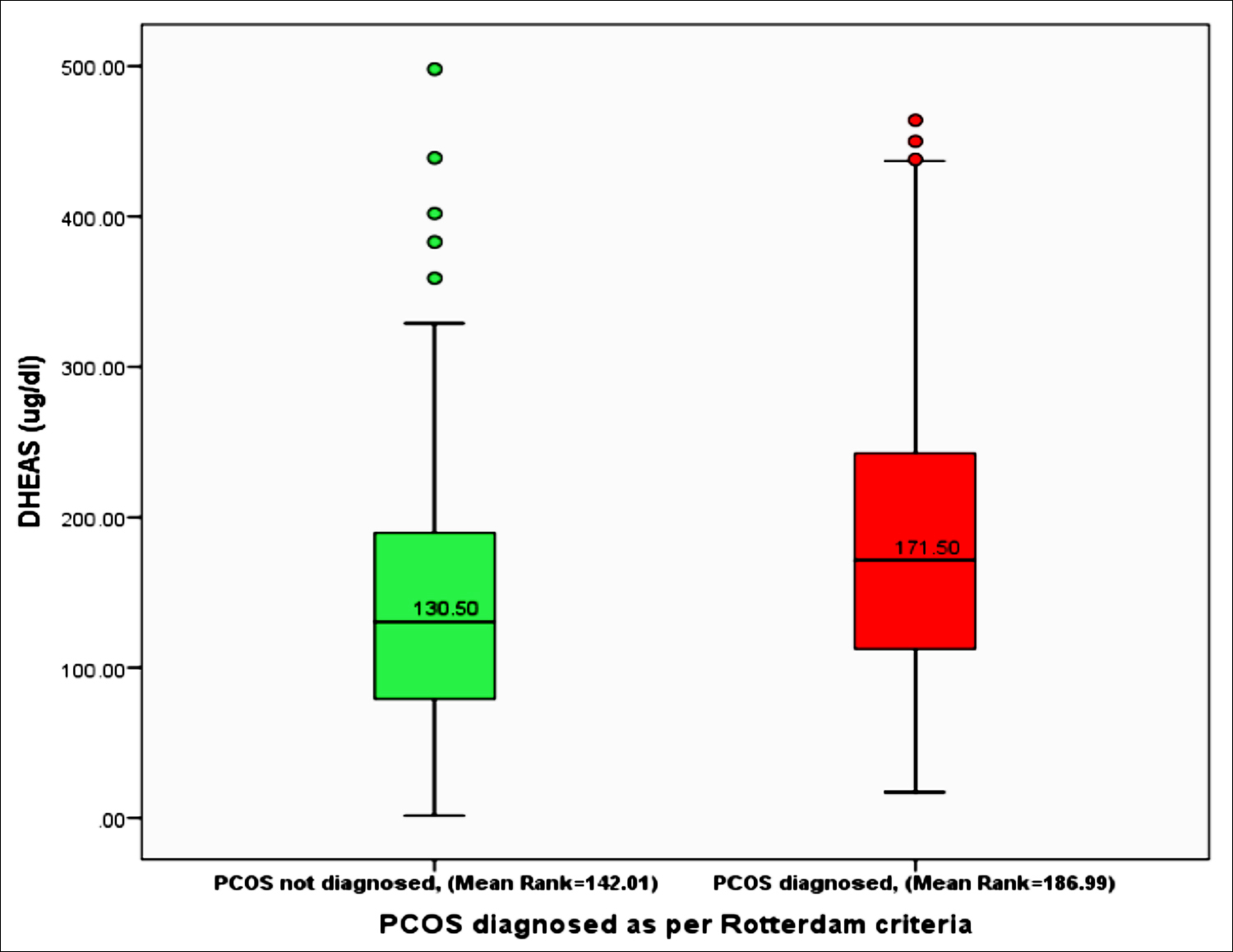 Figure 1: DHEAS levels among subjects with or without PCOS as per Rotterdam diagnostic criteria by utilising non-parametric Mann-Whitney U-test. (n=328, p< 0.001).
Figure 1: DHEAS levels among subjects with or without PCOS as per Rotterdam diagnostic criteria by utilising non-parametric Mann-Whitney U-test. (n=328, p< 0.001).
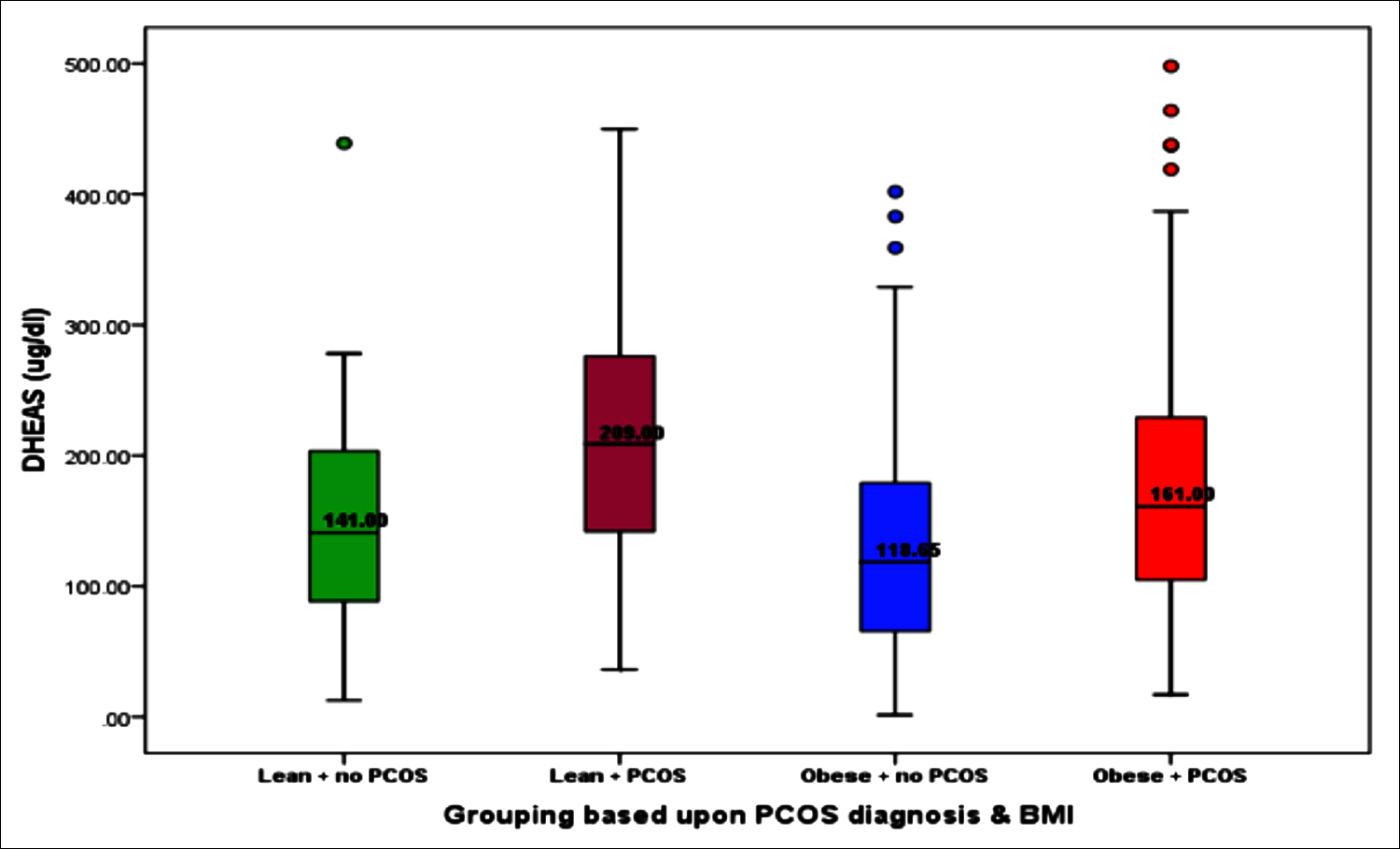 Figure 2: Differences in DHEAS across various groups based upon presence or absence of PCOS as defined criteria of Rotterdam and obesity measured by BMI using Kruskal-Wallis test (p<0.001).
Figure 2: Differences in DHEAS across various groups based upon presence or absence of PCOS as defined criteria of Rotterdam and obesity measured by BMI using Kruskal-Wallis test (p<0.001).
DHEAS levels were found to be inversely related to age (r=-0.218, p<0.001), WHpR (r=-0.099, p=0.073), BMI (r=-0.140, p=0.011), WHtR (r=-0.11, p=0.047), glycemia (r=-0.105, p=0.058), total cholesterol (r=-0.019, p=0.732), triglycerides (r=-0.040, p=0.474), HDLc (r=-0.003, p=0.960), Non-HDLc (r=-0.015, p=0.785), nephropathy (r=-0.048, p=0.386), LH (r=-0.025, p=0.648), FSH (-0.014, p=0.794) and total testosterone (r=-0.003, p=0.959). We observed a rise of DHEAS among lean-PCOS subjects in comparison to lean and obese female subjects with or without PCOS, indicating a major contribution by adrenal androgens in causation of polycystic ovarian morphology as shown in Figure 2.
The difference between insulin resistance as measured by HOMA-IR in subjects categorised based upon presence or absence of obesity (BMI) and PCOS was found to be significant (p=<0.001). The values showed Group-1 with Lean + No PCOS {n=67, 1.78 (1.15-3.07)} vs. Group-2 with Lean + PCOS {n=53, 2.04 (1.09-3.34)} vs. Group-3 with obese + no PCOS {n=96, 3.01 (1.75-4.70)} vs. Group-4 with Obese + PCOS {n=111, 3.20 (2.08-5.64)}.
DISCUSSION
This study has highlighted that adrenal androgens, as evaluated by DHEAS levels, were found to be higher in PCOS and various factors included in the definition of polycystic ovarian syndrome. However, the data does not depict statistical significance for some parameters such as ultrasound presence of PCOS and oligo/anovulation but still DHEAS levels remained higher in subjects with PCOS. These findings are in accordance with the observations highlighted in earlier studies.4,12
The role of DHEAS and insulin resistance in female subjects with lean-PCOS in comparison to obese-PCOS remains differential, where lean-PCOS were observed to have higher DHEAS and low insulin resistance in comparison to obese-PCOS. This is in line with some of the earlier work.12 One study observed DHEAS to be a protective factor in stressful environment; however, the authors also suggested this aspect to be individualised as to their specific mode of stress managing strategy.13 Similarly, a case control studies review have shown low levels of DHEAS to be related with depression, which can be benefitted by administration of DHEAS.14 The above data, therefore, highlights that rise in DHEAS could be physiological phenomena, but some patients could not elicit an appropriate DHEAS response in stress or stress leading to depression. Furthermore, another proof of concept study on MCF-7 cell line (Human metastatic breast cancer cell line) identified DHEAS to have inhibitory role for tumor progression markers like matrix-metalloproteinase 9 and claudin-1, which suggests a protective role of DHEAS in both physiological and pathological stressors including cancers.15 Furthermore, there is literature which highlights DHEAS levels to be negatively correlated with multiple conventional metabolic risks including aging, anthropometric indices, higher lipids, glycemic indices, and hormonal parameters suggesting protective effect related to DHEAS levels.6,7 Similarly, a recent past study has identified better metabolic profiles in subject having raised DHEAS levels; whereas, a meta-analysis has also highlighted that high DHEAS levels, as also observed in this study are associated with better metabolic profile.16,17
Insulin resistance was shown to be higher in subjects with obese-PCOS phenotype in comparison to lean-PCOS type. Reviewed data among PCOS subjects for presence of insulin resistance and metabolic disorders suggested that there are diagnostic and method related issues, which results in variable results in different studies.18 An Asian study highlighted that insulin resistance was associated with PCOS, but the levels at different selected cut-offs were higher in obese PCOS subjects.19 Ageing as an additional factor has been attributed for higher insulin resistance in studied subjects in addition to obesity, where data shows similar trend with age being negatively correlated with DHEAS.20 Apart from obesity, other factors like Omega-3 and vitamin-E have been associated with PCOS.21 However, this study population demonstrated insulin resistance to be more linked to obesity than other PCOS included definition criteria, which resemble the data by Kim et al. and Livadas et al.16,17
Though DHEAS levels remain higher in PCOS in general and with various factors included in PCOS criteria, still its performance as PCOS marker was superseded by modified FG score, FAI, total testosterone levels. Possible reasoning behind this observation could be the phenotypes of PCOS where the DHEAS vary between lean and obese PCOS subjects.12 Feng et al. evaluated serum mannose as a biomarker for diagnosing PCOS in Chinese population in a recent study and showed poor predictive potential of DHEAS along with certain other conventionally utilised biomarkers.22 The present data demonstrated modified FG score for measuring hirsutism severity as the best measure for diagnosis of PCOS, which is also demonstrated by other studies.23 However, contrasting data in literature is available in terms of use of hirsutism as a diagnostic marker for PCOS, as depicted by Wolf et al., who have identified ethnicity and racial differences can confound the use of hirsutism scoring as a diagnostic criteria.24
Taken the findings and shared evidence of this study together, it is not suggested to use DHEAS as clinical biomarker for diagnosing PCOS. However, it is pertinent to explore further the relationship between DHEAS and signaling pathways with links to external environments in association with PCOS.
Certain limitations to this study need to be acknowledged. The prime role of ethnicity and race may be a limiting factor in exact diagnosis of PCOS, which, to some extent, reduce the utility of global use of Rotterdam or any other criteria for diagnosing PCOS. Next limitation could be the need of replicating this cross-sectional analysis on an epidemiological scale for validation purpose.
This study is suggestively clinically significant as it not only highlighted the differential role of DHEAS among lean and obese PCOS, but also signifies the cardio protective effect of DHEAS in terms of its negative correlation with multiple factors including anthropometric profile, lipid biomarkers and reproductive hormones. The cross-sectional study opens up new avenues for research for identifying triggers leading to adrenal androgens and also how lean-PCOS differ from obese-PCOS.
CONCLUSION
DHEAS levels were high in lean-PCOS in comparison to obese-PCOS and non-PCOS females. Furthermore, DHEAS levels were negatively related with various conventional risk factors like BMI, lipid and glucose indices and reproductive hormones.
ETHICAL APPROVAL:
The study ‘’Role of DHEAS in Polycystic Ovarian Syndrome (PCOS)’’ was presented to hospital’s Ethical Committee for review. The committee gave written approval of the study.
PATIENTS’ CONSENT:
All study participants gave a signed written consent before inclusion into study.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
SHK: Correspondence, idea conception, plan, approval of Ethical Review Committee, sampling, Lab analysis, data interpretation, and finalising the manuscript.
SAR: Participant’s examination, and contributed towards discussion.
RS: Patient selection, history and examination, defining Roterdam criteria, contribution to SPSS data analysis.
RM: PCOS gynecological diagnosis, and manuscript writing.
All authors approved the final version of the manuscript.
REFERENCES
- Clark BJ, Prough RA, Klinge CM. Mechanisms of action of dehydroepiandrosterone. VitamHorm 2018; 108:29-73. doi: 10.1016/bs.vh.2018.02.003.
- Prough RA, Clark BJ, Klinge CM. Novel mechanisms for DHEA action. J Mol Endocrinol 2016; 56(3):R139-55. doi: 10.1530/JME-16-0013.
- Goodarzi MO, Carmina E, Azziz R. DHEA, DHEAS and PCOS. J Steroid BiochemMol Biol 2015;:213-25. doi: 10.1016/ j.jsbmb.2014.06.003.
- Blagojevic IP, Eror T, Pelivanovic J, Jelic S, Kotur-Stevuljevic J, Ignjatovic S. Women with polycystic ovary syndrome and risk of cardiovascular disease. J Med Biochem 2017; 36(3):259-69. doi: 10.1515/jomb-2017-0020.
- Yildiz BO, Azziz R. The adrenal and polycystic ovary syndrome. Rev EndocrMetabDisord; 8(4):331-42.
- Abbott DH, Zhou R, Bird IM, Dumesic DA, Conley AJ. Fetal programming of adrenal androgen excess: Lessons from a nonhuman primate model of polycystic ovary syndrome. Endocr Dev 2008; 13:145-58. doi: 10.1159/000134831.
- Lerchbaum E, Schwetz V, Giuliani A, Pieber TR, Obermayer-Pietsch B. Opposing effects of dehydroepiandrosterone sulfate and free testosterone on metabolic phenotype in women with polycystic ovary syndrome. FertilSteril 2012; 98(5):1318-25.e1. doi: 10.1016/j.fertnstert.2012.07.1057.
- Moran C, Arriaga M, Arechavaleta-Velasco F, Moran S. Adrenal androgen excess and body mass index in polycystic ovary syndrome. J ClinEndocrinolMetab 2015; 100(3):942-50. doi: 10.1210/jc.2014-2569.
- Azziz R, Black VY, Knochenhauer ES, Hines GA, Boots LR. Ovulation after glucocorticoid suppression of adrenal androgens in the polycystic ovary syndrome is not predicted by the basal dehydroepiandrosterone sulfate level. J ClinEndocrinolMetab 1999; 84(3):946-50.
- Goodman N, Bledsoe M, Cobin R, Futterweit W, Goldzieher J, Petak S, et al. "American association of clinical endocrinologists hyperandrogenism guidelines". Endocrine Practice 2001; 7(2):120-34.
- Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. FertilSteril 2004; 81(1):19-25.
- Layegh P, Mousavi Z, FarrokhTehrani D, Parizadeh SM, Khajedaluee M. Insulin resistance and endocrine-metabolic abnormalities in polycystic ovarian syndrome: Comparison between obese and non-obese PCOS patients. Int J Reprod Biomed (Yazd). 2016; 14(4):263-70.
- Cho S, Park WJ, Kang W, Lim HM, Ahn JS, Lim DY, Moon JD. The association between serum dehydroepiandrosterone sulfate (DHEAS) levels and job-related stress among female nurses. Ann Occup Environ Med. 2019; 31:e18. doi: 10.35371/ aoem.2019.31.e18.
- Hu Q, Zhang SY, Liu F, Zhang YL, Zhu DM, Zang YY. Clinical significance of decreased protein expression of dehydro-epiandrosterone sulfate in the development of depression: A meta-analysis. J Affect Disord 2015; 174: 416-23. doi: 10.1016/j.jad.2014.11.051.
- Upmanyu N, Bulldan A, Papadopoulos D, Dietze R, Malviya VN, Scheiner-Bobis G. Impairment of the Gnα11-controlled expression of claudin-1 and MMP-9 and collective migration of human breast cancer MCF-7 cells by DHEAS. J Steroid BiochemMol Biol 2018; 182:50-61. doi: 10.1016/j.jsbmb. 2018.04.010.
- Nadir A, Temizkan S, Ozderya A, Temizkan O, Orbay E, Aydin K. Dehydroepiandrosterone sulfate/free androgen index ratio predicts a favorable metabolic profile in patients with polycystic ovary syndrome. Gynecol Endo Crinol 2019; 35(2):128-132. doi: 10.1080/09513590.2018.1505843.
- Wu TT, Chen Y, Zhou Y, Adi D, Zheng YY, Liu F, et al. Prognostic value of dehydroepiandrosterone sulfate for patients with cardiovascular disease: A systematic review and meta-analysis. J Am Heart Assoc 2019; 112(5): 959-966.e1. doi: 10.1016/j.fertnstert.2019.06.035.
- Jeanes YM, Reeves S. Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: Diagnostic and methodological challenges. Nutr Res Rev 2017; 30(1):97-105. doi: 10.1017/S0954422416000287.
- Kim JJ, Hwang KR, Oh SH, Chae SJ, Yoon SH, Choi YM. Prevalence of insulin resistance in Korean women with polycystic ovary syndrome according to various homeostasis model assessment for insulin resistance cutoff values. FertilSteril 2019; 112(5):959-66.e1. doi: 10.1016/ j.fertnstert.2019.06.035.
- Livadas S, Kollias A, Panidis D, Diamanti-Kandarakis E. Diverse impacts of aging on insulin resistance in lean and obese women with polycystic ovary syndrome: evidence from 1345 women with the syndrome. Eur J Endocrinol 2014; 171(3):301-9. doi: 10.1530/EJE-13-1007.
- Rahmani E, Samimi M, Ebrahimi FA, Foroozanfard F, Ahmadi S, Rahimi M, et al. The effects of omega-3 fatty acids and vitamin E co-supplementation on gene expression of lipoprotein(a) and oxidized low-density lipoprotein, lipid profiles and biomarkers of oxidative stress in patients with polycystic ovary syndrome. Mol Cell Endocrinol 2017; 439:247-255. doi: 10.1016/j.mce.2016. 09.008.
- Feng D, Shi B, Bi F, Sagnelli M, Sun X, Jiao J, et al. Elevated serum mannose levels as a marker of polycystic ovary syndrome. Front Endocrinol (Lausanne) 2019; 10: 711. doi: 10.3389/fendo.2019.00711. eCollection 2019.
- Feng JG, Guo Y, Ma LA, Xing J, Sun RF, Zhu W. Prevalence of dermatologic manifestations and metabolic biomarkers in women with polycystic ovary syndrome in north China. J CosmetDermatol 2018; 17(3):511-517. doi: 10.1111/jocd. 12387.
- Wolf WM, Wattick RA, Kinkade ON, Olfert MD. Geographical prevalence of polycystic ovary syndrome as determined by region and race/ethnicity. Int J Environ Res Public Health 2018; 15(11). pii: E2589. doi: 10.3390/ijerph15112589.