CT-guided Percutaneous Radiofrequency Ablation of Osteoid Osteoma with Double Nidi in One Session
By Cennet Sahin1, Eyup Camurcuoglu1, Mehmet Ali Talmac2, Mujdat Bankaoglu1, Kerim Sahin3Affiliations
doi: 10.29271/jcpsp.2020.12.1348ABSTRACT
Osteoid osteoma with multiple nidi is a rare condition. The aim of this report is to share a case of an osteoid osteoma with two separate nidi, which underwent radio-frequency ablation (RFA) treatment under CT-guidance for each of the nidi in the same session. A 15-year girl with osteoid osteoma in left tibia was referred to our clinic for percutaneous RFA. She had pain that worsened at nights. The patient was diagnosed as osteoidosteoma, according to radiologic findings and the clinical symptoms. After CT-guided percutaneous RFA of each nidi in the same procedure, pain was relieved in 24 hours. Each of the RFA-treatments was successful in pain control without any complications and no recurrence occurred during eight months of follow-up period. To our knowledge, this case is one of the very few cases with double nidi, which was treated with RFA in one session. RFA is safe in treatment of osteoid osteomas; even two close and separate nidi can safely be treated in the same session.
Key Words: Osteoid osteoma, Nidi, Treatment, Radiofrequency ablation.
INTRODUCTION
Osteoid osteoma (OO) is a benign bone tumor of young adult males with peak incidence in the second decade of life.1 It is a painful tumor, and is treated with either medical therapy, surgical or radio-frequency ablation (RFA).2,3 RFA has been the first-line treatment option since last 35 years.2,3 OO usually presents with a single nidus, mostly in long extremity bones. OOs with double or multiple nidi are very rare.4-7 Although there are few papers that have reported OOs with multiple nidi, either in a single bone or in separate bones, there are very few cases about percutaneous RFA therapy of an OO with double nidi.6,7 To our knowledge, this is the second case report which is about percutaneous RFA therapy of an OO with double nidi, in which both nidi were ablated separately in the same session.
CASE REPORT
A 15-year female child with an OO was referred to our clinic for percutaneous RFA therapy. She had the lesion on the proximal medial diaphysis of tibia. Her major complaint was pain that was worse, especially at nights for 14 months. She told that pain relieved on taking aspirin.
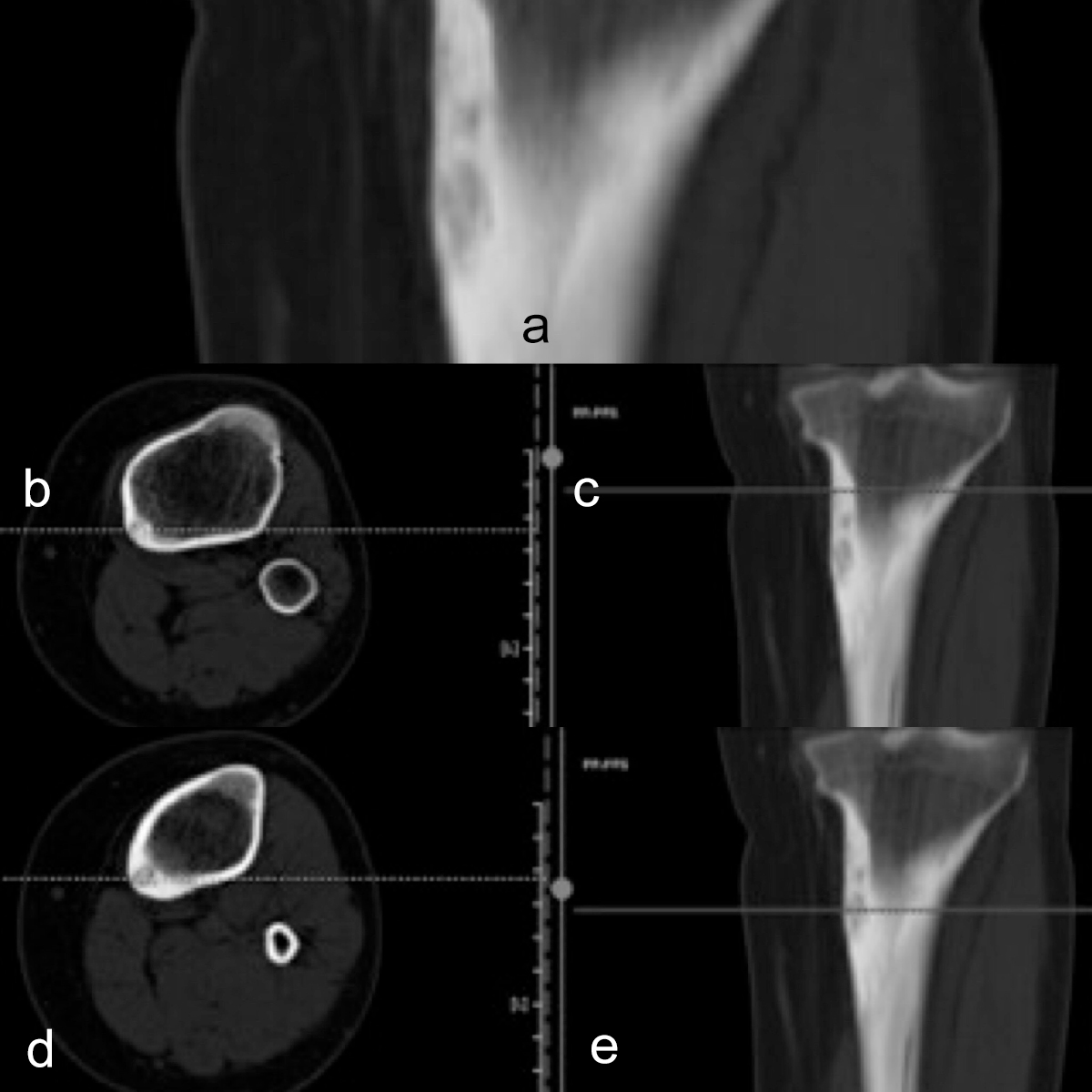 Figures 1 (a-e): On medial side of proximal diaphysis of tibia, asymmetrical cortical thickening is seen on pre-treatment coronal (a) computed tomography image of left lower leg. There are two rounded radiolucencies demonstrating an osteoid osteoma with two separate nidi with 5 mm distance in vertical axis (b-e).
Figures 1 (a-e): On medial side of proximal diaphysis of tibia, asymmetrical cortical thickening is seen on pre-treatment coronal (a) computed tomography image of left lower leg. There are two rounded radiolucencies demonstrating an osteoid osteoma with two separate nidi with 5 mm distance in vertical axis (b-e).
Computed tomography (CT), which was obtained in previous hospital, was evaluated by two radiologists. On CT, there was remarkable focal cortical thickening with two suspicious nidi in medial diaphysis of the tibia (Figure 1). The patient had OO diagnosis based on clinical and CT findings. Since the lesion was suspicious for containing double nidi, magnetic resonance imaging (MRI) was planned for further evaluation. Axial and coronal T2-weighted proton density (PD) MRI (without contrast-medium administration) showed hyperintense double nidi close to each other clearly with surrounding hypointense bone sclerosis without any malignant periosteal reactions. On MRI, there was prominent edema in peri-nidal soft tissue and bone marrow as well (Figure 2). Because the patient had no clinical symptoms or laboratory changes (sedimentation, hemogram, C-reactive protein), suspicious for infection or malignancy, definitive diagnosis of OO was made.
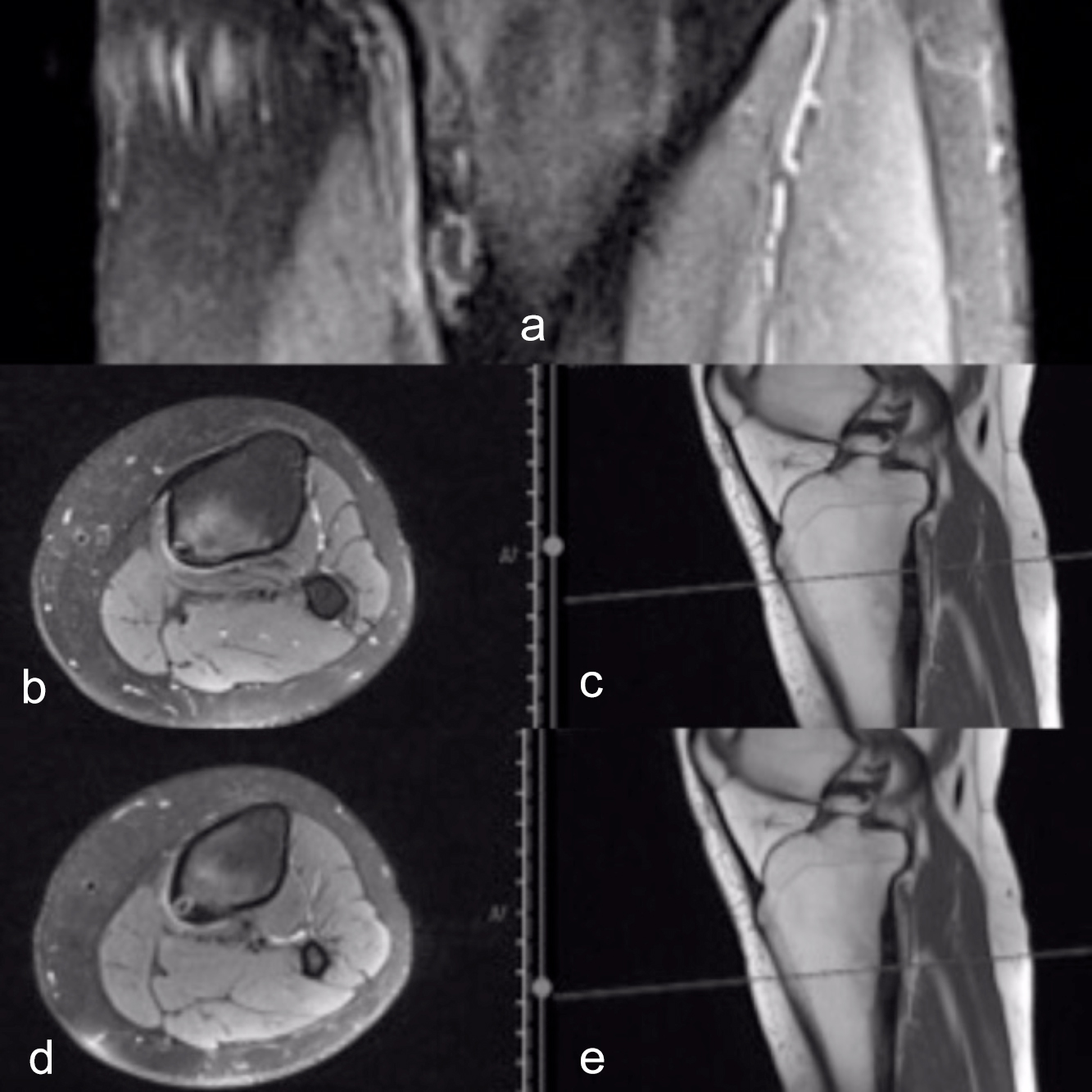 Figures 2 (a-e): Pre-procedural T2-weighted coronal (a), axial (b, d) and sagittal (c, e) MRIs, without contrast medium. There are two nidi close to each other in vertical axis (a). Both the nidi are signal void with hyperintense rim on axial Proton-density (PD) MRIs (b, d). Due to perinidal bone-marrow and soft tissue edema, high-signal intesity is seen clearly on MRIs (a, b, d).
Figures 2 (a-e): Pre-procedural T2-weighted coronal (a), axial (b, d) and sagittal (c, e) MRIs, without contrast medium. There are two nidi close to each other in vertical axis (a). Both the nidi are signal void with hyperintense rim on axial Proton-density (PD) MRIs (b, d). Due to perinidal bone-marrow and soft tissue edema, high-signal intesity is seen clearly on MRIs (a, b, d).
Percutaneous RFA under CT-guidance was planned for each nidus in the same session. Institution’s Ethical Committee approval was obtained for retrospective evaluation of the RFA therapies performed in the institution (Number: 1879; dated: 23.01.2018). Informed consent for the probable complications related to either RFA or anesthesia and to publish the data was obtained from the patient’s parents.
Two CT-guided RFA treatments were performed separately for each nidus in the same session by two radiologists. Both RFA therapies were performed under spinal anesthesia and superficial sedation in December 2018. Anesthesia was administered by an experienced anesthesiologist. One hour before the procedure, cephalexin (25 mg/kg; intravenous) was administrated for prophylaxis. Since the RFA electrode was monopolar, two grounding pads were placed on both lower extremities. A coaxial-needle (12 G, 10 cm; AprioMed, Sweden) was placed into the superior nidus firstly under CT-guidance (Toshiba, Alexion, Japan). A bone biopsy specimen was obtained through this coaxial needle with a 14 G 12.5 cm bone needle (Ostycut, BARD, USA). The RFA electrode (Angio-Dynamics, USA) was placed into the nidus through coaxial-needle (Figure 3). Prior to heating, after placement of the RFA probe, hydro-dissection was performed with 20 ml of 5% dextrose solution to prevent skin burn in the subcutaneous fatty tissue around the coaxial needle. Additionally, concerning about possible skin burns, cold ice cup in a sterile towel was applied around the needle on to the skin during the process. Since both of nidi were not very close to skin with a 4 cm of soft tissue thickness, we planned to perform two separate RFA therapies for each nidus in the same session. The RFA electrode was heated up to a maximum of 80°C for 5 minutes for each therapy. Similar procedures of biopsy (that revealed OO diagnosis histopathologically) and RFA were also performed for the second nidus, which was localized 5 mm inferior to the first-one. The procedures were completed successfully and safely. No complication occurred related to the treatments (Figure 4).
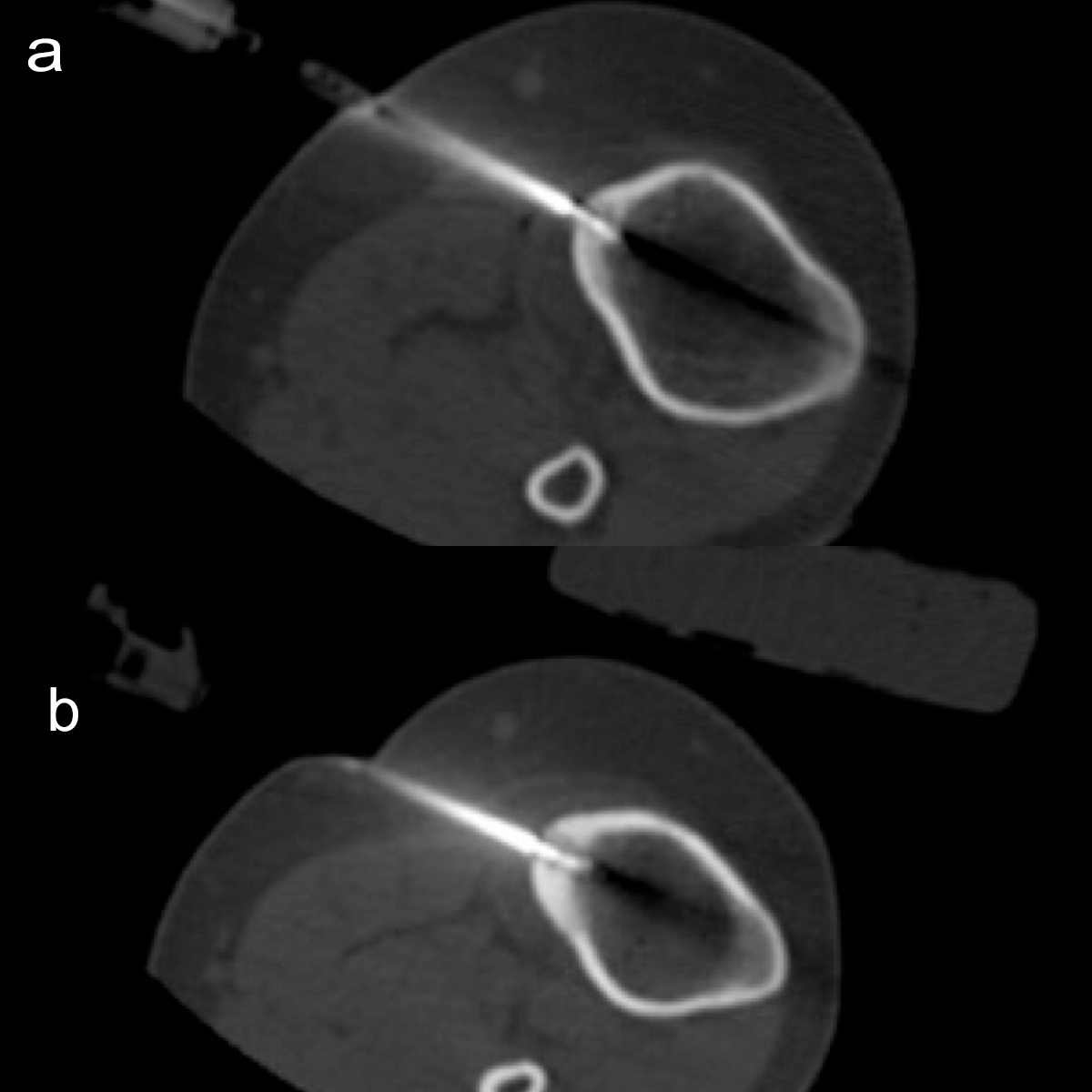 Figures 3 (a,b): On computed tomography images obtained during treatment, coaxial-needles and the RFA-electrodes are seen for each procedure for each of the nidi separately.
Figures 3 (a,b): On computed tomography images obtained during treatment, coaxial-needles and the RFA-electrodes are seen for each procedure for each of the nidi separately.
The patient was hospitalised in Orthopedics Department for one night for close follow-up for any potential complications. The intervened skin area on tibia was normal without any skin burn, edema or erythema. Pain (that was due to OO) was totally relieved on the next day of follow-up. When the patient was re-assessed one week later, she was painless without any complaint or skin problem. On the follow-up MRI(s) on 6th month, comparing the imaging findings of cortical thickness degree, sizes of the both nidi and perilesional soft tissue and bone marrow edema with pre-treatment MRIs, there was a slight regression in imaging findings (Figure 5).
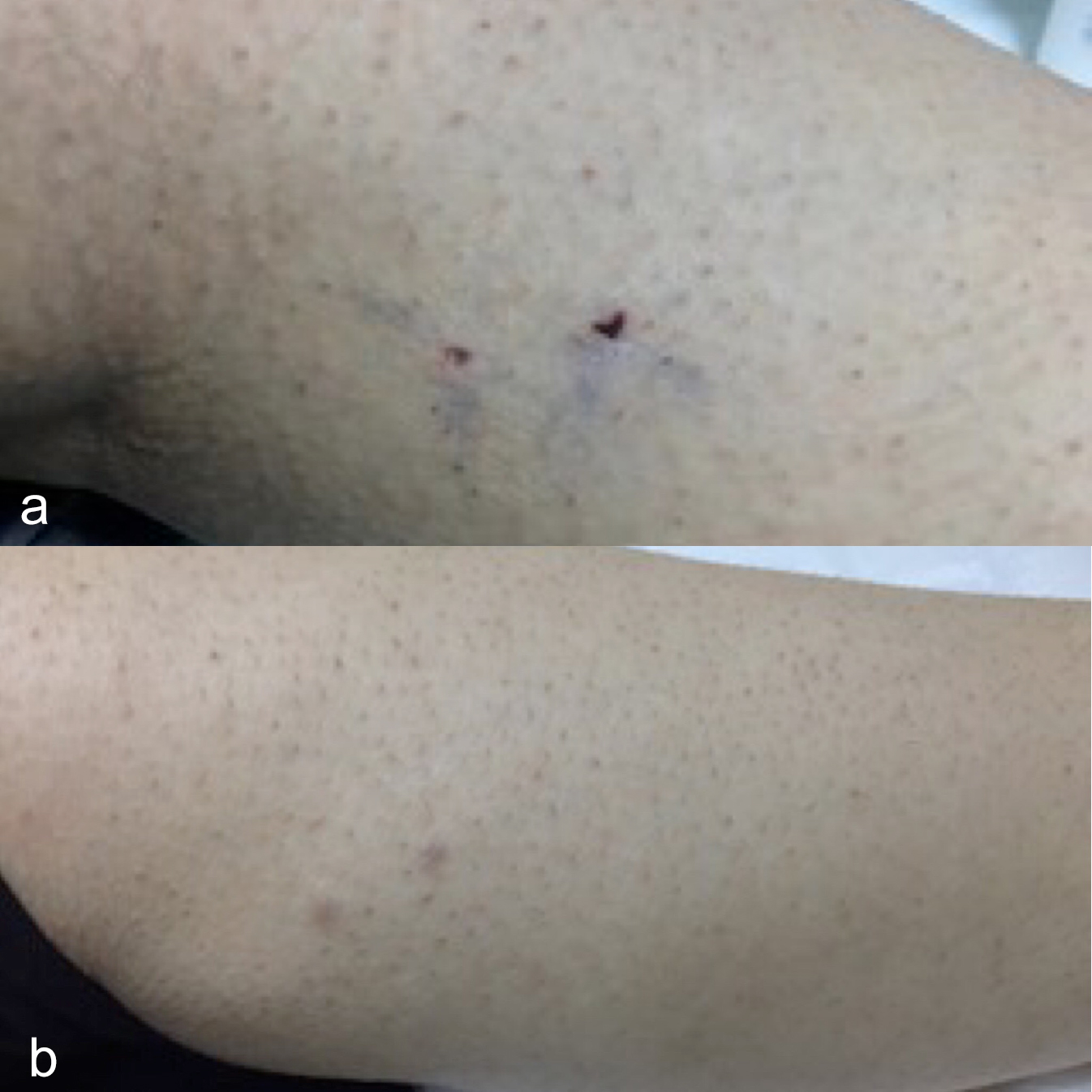 Figures 4 (a,b): Photos of the extremity at first week (a) and 6th month (b) follow-ups. After the ablations, there was no burn on the intervened area of the skin.
Figures 4 (a,b): Photos of the extremity at first week (a) and 6th month (b) follow-ups. After the ablations, there was no burn on the intervened area of the skin.
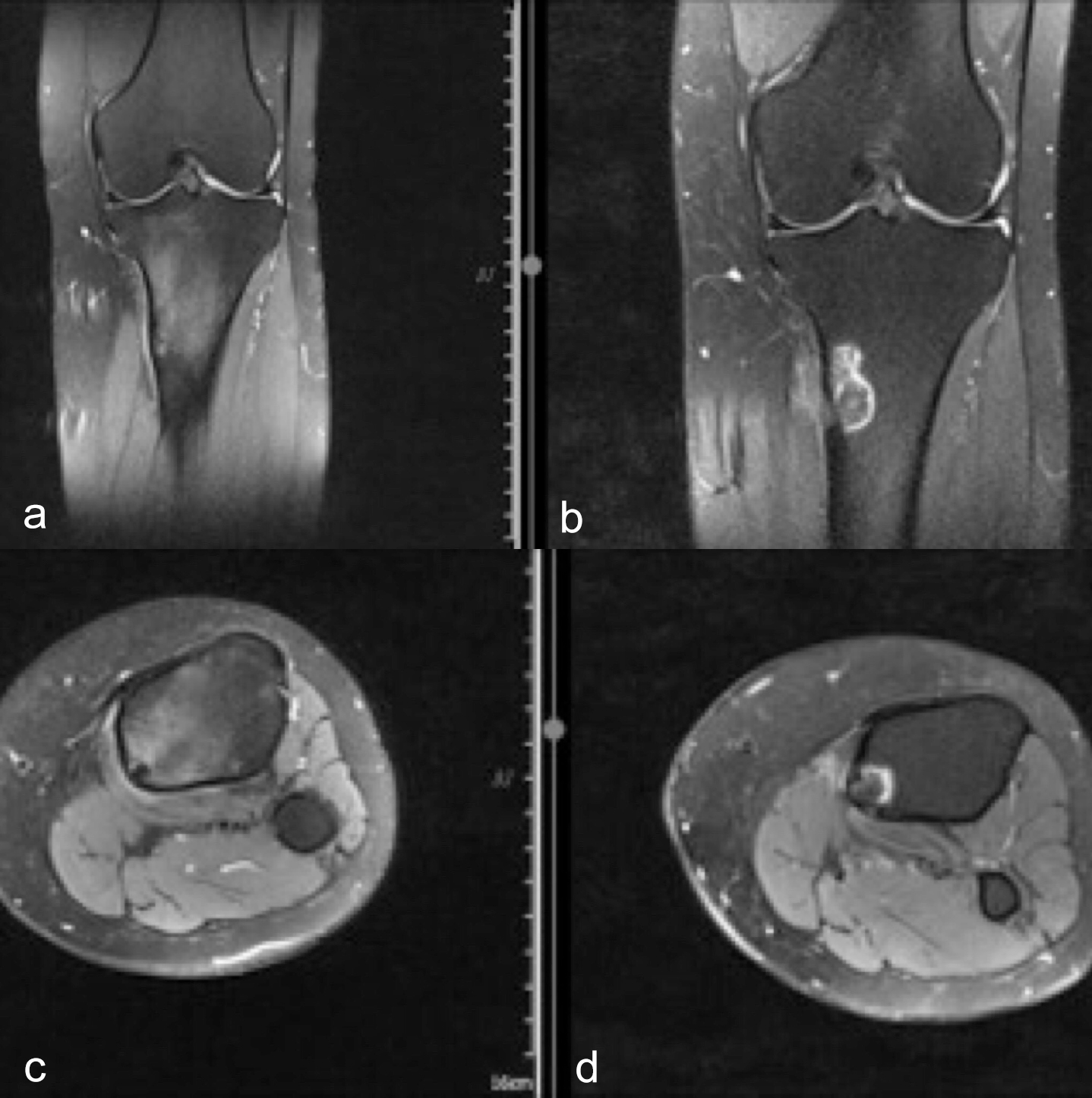 Figure 5 (a-d): On follow-up MRIs on 6th month (b, d), regression in edema in bone marrow and soft tissue is seen compared to pre-treatment MRIs (a, c). Geographic shaped hyperintensity in bone marrow through the needle track occurred due to necrosis that was caused by ablation zone (b, d). The hyperintensity on T2-weighted MRIs through needle tracks and ablation zones in bone marrow, that was not present in pre-treatment images, should not be misdiagnosed as worsening of radiologic findings.
Figure 5 (a-d): On follow-up MRIs on 6th month (b, d), regression in edema in bone marrow and soft tissue is seen compared to pre-treatment MRIs (a, c). Geographic shaped hyperintensity in bone marrow through the needle track occurred due to necrosis that was caused by ablation zone (b, d). The hyperintensity on T2-weighted MRIs through needle tracks and ablation zones in bone marrow, that was not present in pre-treatment images, should not be misdiagnosed as worsening of radiologic findings.
DISCUSSION
OO usually presents with a single nidus.1 Presentation of an OO with multiple nidi is very rare. Previous studies have reported cases of OO with multiple nidi in a single bone or in separate bones.4-7 Although there are several cases of OO with multicentric or double nidi (in a single bone or in separate bones) in the literature, to our knowledge, this is the second case which had two percutaneous RFA therapies for each of the double nidi in an OO in the same session.7 As in our case, Miyazaki et al. have reported a case which was about RFA of an OO with two nidi in one session under fluoroscopy-guidance.7 Giuseppe et al. reported repeated RFA therapy for metachronous OOs that presented 34 years later in different localisations in the same patient.5 Kaul et al. have published a case which was about percutaneous laser therapy of an OO with double nidi under MR-guidance.6
It is well-known that CT is superior to other radiological methods in the diagnosis of OO.6 After evaluating the CT (that was obtained in previous hospital), we noticed that there were two different radiolucent nidi with uniformly sharp sclerotic rim in the proximal medial diaphysis of left tibia. Since this was a rare condition, we performed MRI for further evaluation. MRI with PD-weighted sequences, revealed presence of two separate nidi in one OO. Bone marrow and soft tissue edema supported our diagnosis. The most common symptom of an OO is pain and major reason for treatment is pain management.1-3 Since, the most prominent symptom was pain, separate treatments were planned for each of the two nidi.
Efficacy and safety of RFA in OO and its advantages compared to surgical therapy are well known.2,3 But, due to close proximity of both nidi to each other and moderate proximity to the skin, we had a hesitation about probable skin burn due to multi-RFA therapies for the two nidi in the same session. We overcame this problem by preventing skin burn through hydro-dissection of subcutaneous soft tissue by injecting 20 ml of 5% dextrose and also ice application around the intervening area during RFA(s). Although, we usually heat the RFA probe up to 90° C for 7 minutes in our routine procedures; the RFA electrode was heated up to a maximum of 80° C for 5 minutes in this case. Owing to these precautions, no skin burn or any other complication was seen related to the procedure. Pain relief occurred in 24 hours. Although the procedure was clinically successful, we wanted to see the success with follow-up MRI. The healing of perinidal edema was seen clearly in 6th months’ follow-up MRI.
Reviewing the limited numbers of OO patients with multiple nidi, our case is worthy of dissemination. Our case had a successful result after only one procedure for each of the two nidi, and no recurrence occurred in the follow-up period of 8 months. We aim to highlight the differential diagnosis of an OO with multiple nidi and the safety of multiple percutaneous ablation treatments even for two nidi in close proximity in the same OO in one session.
As a limitation of this study, this was the only case which underwent double RFA treatment in the same session among 160 cases of OO in our series, since OO with double nidi is extremely rare. Although, eight months of follow-up time is enough for such a benign tumor, it is shorter than one year. Longer follow-up is required to see the long-term efficacy of the RFA in this patient.
Different treatment options with either RFA, microwave or laser may be used in future cases to compare the success and efficacy of the percutaneous treatment of OO(s) with double nidi.
In conclusion, CT-guided percutaneous RFA treatments of OO with multi nidi in one session is safe, minimally invasive and effective. As both of the RFA treatments were successful in pain control in this patient and no recurrence has occurred during 8-month follow-up period, we wanted to add our case report to the existing knowledge-base with this article.
PATIENT’S CONSENT:
Informed consent for the probable complications related to either RFA or anesthesia and to publish the data was obtained from the patient’s parents.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
CS: Evaluated the case with detailed history taking and radiological examinations, performed the intervention, reported and supervised the manuscript, made correspondence.
EC: Assisted the intervention and contributed to design of the work, data collection and literature review.
MB: Contributed to the radiological diagnosis and conception of the work.
MAT: Performed physical examination of the case; contributed to the design of the work and data collection.
KS: Performed the anesthesia and revised the content of the work.
REFERENCES
- Iyer RS, Chapman T, Chew FS. Pediatric bone imaging: Diagnostic imaging of osteoid osteoma. AJR Am J Roentgenol 2012; 198(5):1039-52. doi: 10.2214/AJR. 10.7313.
- Hage AN, Chick JFB, Gemmete JJ, Srinivasa RN. Percutaneous radiofrequency ablation for the treatment of osteoid osteoma in children and adults: A comperative analysis in 92 patients. Cardiovasc Intervent Radiol 2018; 41(9):1384-90. doi: 10.1007/s00270-018-1947-7.
- Lassalle L, Campagna R, Corcos G, Babinet A, Larousserie F, Stephanazzi J, et al. Therapeutic outcome of CT-guided radiofrequency ablation in patients with osteoid osteoma. Skeletal Radiol 2017; 46(7):949-56. doi: 10.1007/s00256-017-2658-x.
- Tamam C, Yildirim D, Tamam M. Multicentric osteoid osteoma with a nidus located in the epiphysis. Pediatr Radiol 2009; 39(11):1238-41. doi: 10.1007/s00247- 009-1363-x.
- Giuseppe C, Andrea CD, Rodolfo C. Metachronous osteoid osteoma thirty-four years later: Case report. J Clin Case Rep 2013; 4:327. doi:10.4172/2165-7920.1000327.
- Kaul D, Bonhomme O, Schwabe P, Gebauer B, Streitparth F. Osteoid osteoma with a multicentric nidus: Interstitial laser ablation under MRI guidance. Case Rep Orthop 2013; 2013:254825. doi: 10.1155/2013/254825.
- Miyazaki M, Miyazaki A, Kurabayashi T, Shinozaki T, Endo K, Tsushima Y. CT fluoroscopy-guided radiofrequency ablation of osteoid osteoma with double NIDI. Jpn J Radiol 2012; 30(1):78-80. Doi.10.1007/s11604-011-0012-0.