CROSS or FLOT in Distal Esophageal and Gastroesophageal Cancer
By Ibrahim Karadag, Serdar Karakaya, Ozturk Ates, Omur Berna Cakmak OksuzogluAffiliations
doi: 10.29271/jcpsp.2021.03.326ABSTRACT
Objective: To compare the chemoradiotherapy for esophageal cancer followed by surgery study (CROSS) and continuous infusion 5-FU, leucovorin, oxaliplatin, and docetaxel (FLOT) protocols administered in distal esophageal and gastroesophageal junction (GEJ) tumors in terms of effectiveness and toxicity.
Study Design: Descriptive study.
Place and Duration of Study: Ankara Oncology Training and Research Hospital, Turkey between 2015 and 2020.
Methodology: Patients diagnosed with distal esophageal and GEJ squamous cell carcinoma (SCC) or adenocarcinoma (ADC), older than 18 years of age, in localised or locally advanced stage were included. Metastatic stages were excluded. Kaplan-Meier was used for survival analysis, log-rank test was performed for comparisons between groups.
Results: A total of 25 patients (44.6%) were treated with CROSS protocol (15 distal esophageal and 10 GEJ tumor), 31 patients (55.4%) with GEJ tumors were treated with the FLOT regimen. Eight of the patients who were administered the CROSS protocol before the operation demonstrated complete pathologicial response, no patients in the FLOT group had complete response to the treatment. In patients with GEJ tumors and ADC histopathology, CROSS and FLOT group had similar second years survival (60% and 59.3%, respectively) (p = 0.803). The frequency of neutropenia was significantly higher in the CROSS group compared to the FLOT group (p = 0.004.)
Conclusion: Postoperative pathological response rate in the CROSS group was significantly higher compared to the FLOT group. CROSS and FLOT protocols contributed to survival similarly in patients with GEJ ADC, hematological side effects were more pronounced in patients receiving CRT.
Key Words: GEJ cancer, Esophageal cancer, Cross, Flot.
INTRODUCTION
Esophageal cancer is a common malignancy, causing more than 400,000 deaths annually worldwide.1,2 According to the Siewert classification based on the localisation of GEJ tumors, distal esophageal tumors are classified as Siewert Type I, junction tumors are classified as Type II and proximal gastric tumors are classified as Type III.3,4 According to the current AJCC TNM staging system (eighth edition, 2017), proximal stomach tumors less than 2 cm away from the GEJ and tumors involving the GEJ are considered esophageal cancers, while tumors with a GEJ infiltration and those with a midpoint extending more than 2 cm into the stomach are considered gastric cancer.5
OS is still poor in cancers of the esophagus and GEJ. Better results have been obtained with multimodal treatment approaches such as CT and/or CRT in localised and locally advanced cancers.6-9 In CROSS study, surgery after neoadjuvant CRT in SCC and ADC patients was compared to treatment with surgery only. A higher R0 resection rate and better OS were achieved with neoadjuvant CRT (48 vs. 24 months).10 Surgery after CRT has become standard in many centers, since CRT completion rates are quite high and side effects are low.
In the FLOT4 study, which is another important research involving patients diagnosed with locally advanced, resectable gastric or GEJ adenocarcinoma, 56% of randomised patients had GEJ carcinoma. A 50-month OS advantage was demonstrated in patients undergoing FLOT regimen (docetaxel, oxaliplatin, leucovorin with short-term infusional fluorouracil) for 4 cycles.11 Based on these data, the perioperative FLOT regimen has been increasingly preferred especially in medically fit patients with GEJ tumors. Treatment of distal esophageal and GEJ tumors is controversial and many different studies are currently being undertaken to determine a standardizsed approach to treatment.
The main aim of this study was to compare the CROSS and FLOT protocols administered in distal esophagus and GEJ tumors at our oncology center in terms of effectiveness and toxicity.
METHODOLOGY
Patients diagnosed with distal esophageal and GEJ carcinoma in the medical oncology clinic of Ankara Oncology Training and Research Hospital between 2015 and 2020 were retrospectively reviewed. Fifty-six patients older than 18 years of age, diagnosed with SCC or ADC in localised or locally advanced stage were included. Metastatic stages were excluded. Patients who were administered the CROSS protocol (41 Gy radiotherapy with 2 mg/ml/min carboplatin and 50 mg/m2 paclitaxel, weekly) and FLOT regimen given in the form of 4 cycles perioperatively (50 mg/m2docetaxel plus 85 mg/m2 oxaliplatin and 200 mg/m2 leucovorin with 2600 mg/m2 infusional fluorouracil as a 24-hour infusion, all on day 1, administered every two weeks) were evaluated. The data were collected retrospectively from the database of this research hospital. OS was calculated according to the date of death reported in the central registry (death notification form). The study was approved by the local Ethics Committee (23.6.2020 approval No. 97).
Statistical analyses were performed with the SPSS version 25.0 software (SPSS, Chicago, IL, USA). Categorical variables were presented as number (n) and percentage (%) values. Continuous variables were presented as median (IQR) (Interquartile Range) accordingly. Chi-square test was used to compare categorical data. The Kaplan-Meier method was used for survival analysis and the log-rank test was performed for comparisons between groups. A value of p<0.05 was considered statistically significant.
RESULTS
Demographic, histopathological features, type of treatment and treatment responses of patients are summarised in Table I.
Table I: Patient characteristics.
|
Characteristics |
CROSS |
FLOT |
All patients (n) |
|
Patients |
25 |
31 |
56 |
|
Female |
10 (40%) |
6 (19.4%) |
16 %28.6%) |
|
Male |
15 (60%) |
25 (80.6%) |
40 (71.4%) |
|
Median age |
57 (51-69.5) |
63 (54-69) |
60 (53-69) |
|
Histology Adeno SCC |
10 (40%) 15 (60%) |
31 (100%) 0 |
41 (73.2%) 15 (26.8%) |
|
Lymph node positivity |
17 (68%) |
29 (93.5%) |
46 (82.1%) |
|
Operation |
21 (84%) |
19 (%61.3) |
40 (71.4%) |
|
ypT category T0 T1 T2 T3 T4 |
7 (28%) 4 (16%) 5 (20%) 1 (4%) 1 (4%) |
2 (6.5%) 2 (6.5%) 0 13 (41.9%) 2 (6.5%) |
|
|
ypN category N0 N positivitiy |
14 (56%) 6 (24%) |
4 (12.9%) 15 (48.5%) |
|
|
Pathologic response Complete Partial Unresponsive/Minimal |
8 (32%) 11 (44%) 1 (4%) |
0 10 (32.2%) 9 (29%) |
|
|
Recurrence |
4 (16%) |
4 (12.9%) |
|
Median OS was 53 months (95% C.I: 14.0 – 92.0 months). The estimated survival in the first and second years was respectively 90.9% and 75.5% in the CROSS group, and 88.9 and 59.3% in the FLOT group (p = 0.366). The median OS of patients with tumors located in the distal esophagus and had SCC histopathology was 58 months, and the median OS of patients with GEJ tumors and had ADC histopathology was 53 months (95% C.I: [3.9-102.1] moths) and there was no statistical difference between them (p = 0.326). In patients with GEJ tumors and ADC histopathology, CROSS recipients had an estimated survival of 75% and 60% in the first and second years, respectively. In the FLOT group, these values were 88.9% and 59.3%, respectively (p = 0.803) (Figure 1). The hematologic side effect profiles are summarized in Table II.
Table II: Summary of hematological adverse events in patients receiving CROSS or FLOT.
|
Adverse event |
CROSS |
FLOT |
p-value |
|
Anemia Grade 1-2 Grade 3-4 |
20 (80%) 0 |
17 (54.8%) 0 |
0.053 |
|
Neutropenia Grade 1-2 Grade 3-4 |
12 (48%) 3 (12%) |
3 (9.7%) 3 (9.7%) |
0.004 |
|
Thrombocytopenia Grade 1-2 Grade 3-4 |
9 (36%) 0 |
5 (16.1%) 0 |
0.231 |
|
Delayed treatment |
10 (40%) |
11 (35.5%) |
0.729 |
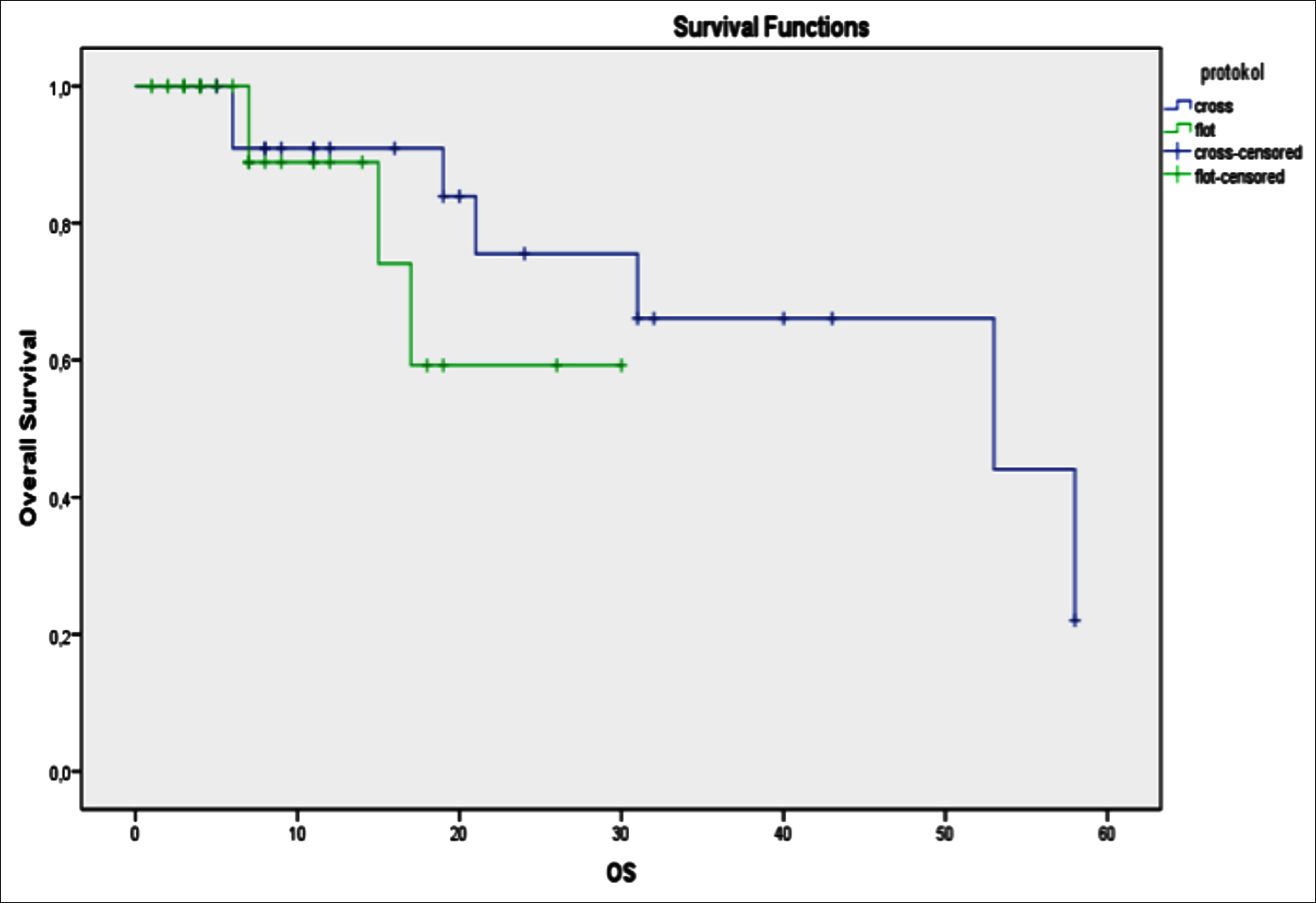 Figure 1: Overall survival in patients by the treatment regimens.
Figure 1: Overall survival in patients by the treatment regimens.
DISCUSSION
In this retrospective study comparing the effectiveness of CROSS and FLOT regimens in distal and junctional esophageal CA, postoperative pathological response rates were significantly better in the CROSS group than in the FLOT group. In addition, after neoadjuvant therapy, 84% (n = 21) of the CROSS group could be operated while this percentage was 61.3% (n = 19) in the FLOT group. In this study, median OS was 53 months. The estimated two-year survival was 75% in the CROSS group and 59.3% in the FLOT group.
In the CROSS study, which published long-term results in 2015 by Shapiro et al., the results of all patients with esophageal and GEJ tumors with SCC and ADC histopathology were compared with regard to treatment characteristics.10 At the 84-month follow-up, median OS was reported to be 48.6 months in all patients receiving neoadjuvant CRT, while patients with SCC and ADC had OS values of 81.6 and 43.2 months, respectively.
In this study, all 15 patients with SCC had distal esophageal tumors and the median OS in the SCC group was 58 months. In contrast, the SCC subgroup of the pivotal CROSS study had a median OS of 81 months. Differences in ethnicity and tumor localisation of patient groups (58% of patients [SCC + ADC] had distally located tumors in the CROSS study) and the longer median follow-up duration compared to our study (84 vs. 10 months),12 may be listed among the causes of this difference.
In this study, tumors of all patients with ADC histopathology (73.2%, n = 41) were located in the GEJ and the median OS of these patients was 53 months. In contrast, the CROSS study had a total of 134 patients with ADC tumors located in the distal esophagus and GEJ. The median OS of these patients was 43.2 months. Different tumor location and thus differences in biological behavior and treatment response may have led to this between this study and CROSS trial.
In the FLOT4 study by Al-Batran et al., 200 (56%) patients had GEJ tumors of which 85 (24%) were Siewert type 1 and 115 (32%) were Siewert type 2-3.11 At the end of the 43-month median follow-up, the median OS of gastric and GEJ tumors was determined to be 50 months. In our study, 31 GEJ ADC patients had received the FLOT regimen and their estimated two-year survival was 59%. In a retrospective study involving patients with distal esophagus and Siewert type-1 ADC, 53 patients received preoperative CROSS regimen, while 51 patients received perioperative EOX (epirubicin, oxaliplatin, capecitabine) treatment. The six-month and one-year median OS was significantly higher in the periopreative CT group compared to the CRT group (92% vs 85% in the sixth month, 75% vs. 66% in the first year, p <0.001).13
The present results showed that the postoperative pathological response rate in the CROSS group was significantly better compared to the FLOT group. While complete pathological response frequency was 32% (n = 8) in the CROSS group, none of the patients in the FLOT group had complete response to treatment. This may be due to the fact that patients in the FLOT group had tumors in more advanced stages at the time of diagnosis compared to those in the CROSS group. Clinical lymph node positivity was present in 93.5% of the patients who were given FLOT regimen at the time of diagnosis.
In another retrospective study, consistent with these results, pathological complete response rate in the CROSS group was higher than in the FLOT group (12% vs. 5%, respectively; p =0.001).13 In a study conducted by Hopner et al. on esophageal ADC, it was shown that the group receiving preoperative CRT had better pathological response rates than the group receiving perioperative CT. But this result was not reflected in OS rates which were similar in the two groups.14 In another study comparing the perioperative CT (epirubicin, capecitabine, cisplatin) treatment with the CROSS protocol, the estimated 3-year survival was determined to be similar (50% vs. 49%, respectively; p = 0.93).15
When previous retrospective studies and our findings are evaluated together, it seems evident that preoperative CRT provides better pathological response and locoregional control compared to CT. But it is difficult to suggest the same for OS. In one study, better OS rate was detected in perioperative CT recipients; whereas, in other studies similar OS contributions were detected. In our study, although the number of patients with GEJ ADC was low, the estimated two-year survival rates of the CROSS and FLOT groups were similar. All hematological side effects were at a higher frequency in the CROSS group in our study but there was no significant difference between two groups in terms of delay in treatment due to side effects.
This study has some limitations. It was retrospective, prospective multicenter study would be much better in terms of evaluating the effectiveness and reliability of CROSS and FLOT regimens. In this study, there is a risk of bias in some results due to the low number of patients and missing data.
CONCLUSION
CROSS and FLOT protocols contribute similarly to survival outcome in patients with GEJ ADC, and hematological side effects are more pronounced in patients receiving CRT. Large prospective studies on this subject will provide better information and could reduce the possibility of bias.
ETHICAL APPROVAL:
This study was conducted in compliance with the ethical principles according to the Declaration of Helsinki, and it was approved by the local Institutional Review Board (No. 2020/97).
PATIENTS’ CONSENT:
Since it was designed as a retrospective study, the data were collected from the Hospital archive after approval of the Ethics Committee.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
IK, SK, OA, OBO: Conceived the study design, involved in data collection, performed the statistical analysis, interpreted data and prepared the manuscript draft.
All the authors critically reviewed the final version of the manuscript and approved the final version.
REFERENCES
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6):394-424. doi: 10.3322/caac.21492.
- Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol 2016; 41: 88-95. doi: 10.1016/j.canep.2016.01.013.
- Siewert J, Hölscher A, Becker K and Gössner W. Cardia cancer: Attempt at a therapeutically relevant classification. Der Chirurg Zeitschrift fur alle Gebiete der operativen Medizen 1987; 58(1):25-32.
- Siewert J and Stein H. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998; 85(11): 1457-9. doi: 10.1046/j.1365-2168.1998.00940.x.
- Amin MB and Edge SB. AJCC cancer staging manual. springer 2017;
- Lutz MP, Zalcberg JR, Ducreux M, Ajani JA, Allum W, Aust D, et al. Highlights of the eortc st. gallen international expert consensus on the primary therapy of gastric, gastroesophageal and oesophageal cancer–Differential treatment strategies for subtypes of early gastro-esophageal cancer. Eur J Cancer 2012; 48(16):2941-53. doi: 10.1016/j.ejca.2012.07.029.
- Tulay E, Karacin C, Gokhan U, Ergun Y, Yazici O, Imamoglu GI, et al. Efficacy of the combination of modified docetaxel, cisplatin and fluorouracil in locally advanced gastric cancer: Evaluation of real-life outcomes. UHOD 29: 001-9.
- Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007; 25(10):1160-8. doi: 10.1200/JCO.2005.04.7118.
- Shridhar R, Freilich J, Hoffe SE, Almhanna K, Fulp WJ, Yue B, et al. Single-institution retrospective comparison of preoperative versus definitive chemoradiotherapy for adenocarcinoma of the esophagus. Ann Surg Oncol 2014; 21(12):3744-50. doi: 10.1245/s10434-014-3795-2.
- Shapiro J, Van Lanschot JJB, Hulshof MC, van Hagen P, van Berge Henegouwen MI, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015; 16(9):1090-8. doi: 10.1016/S1470-2045(15) 00040-6.
- Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019; 393 (10104): 1948-57. doi: 10.1016/S0140-6736(18)32557-1.
- Siewert JR and Ott K. Are squamous and adeno-carcinomas of the esophagus the same disease? Semin Radiat Oncol 2007; 17(1):38-44. doi: 10.1016/ j.semradonc.2006.09.007.
- Koch OO, Weitzendorfer M, Varga M, Tschoner A, Partl R, Perathoner A, et al. Perioperative chemotherapy versus neoadjuvant chemoradiation for patients with adenocarcinoma of the distal esophagus in Austria: a retrospective analysis. World J Surg Oncol 2019; 17(1):146. doi: 10.1186/s12957-019-1693-6.
- Hoeppner J, Zirlik K, Brunner T, Bronsert P, Kulemann B, Sick O, et al. Multimodal treatment of locally advanced esophageal adenocarcinoma: Which regimen should we choose? Outcome analysis of perioperative chemotherapy versus neoadjuvant chemoradiation in 105 patients. J Surg Oncol 2014; 109(3):287-93. doi: 10.1002/ jso.23498.
- Goense L, van der Sluis PC, van Rossum PS, van der Horst S, Meijer GJ, Haj Mohammad N, et al. Perioperative chemotherapy versus neoadjuvant chemoradiotherapy for esophageal or GEJ adenocarcinoma: A propensity score‐matched analysis comparing toxicity, pathologic outcome, and survival. J Surg Oncol 2017; 115(7):812-20. doi: 10.1002/jso.24596.