Can COVID-19 Cause Pancreatitis? A Rare Complication of SARS-CoV-2 Infection
By Muhammad Hanif1, Abdul Wali Khan1, Sadiq Ullah2, FNU Sundas1, Sheraz jamal Khan1Affiliations
doi: 10.29271/jcpsp.2021.07.120ABSTRACT
Coronavirus disease-2019 (COVID-19) emerged as a cluster of atypical pneumonia in Wuhan, China in December 2019. This disease has been declared a pandemic by the World Health Organization. COVID-19 patients mostly present with upper respiratory symptoms like dyspnea, cough and fever. Various neurological, myocardial, renal and gastrointestinal complications have been reported associated with SARS-CoV-2. Acute pancreatitis is one of the common causes of upper abdominal pain, caused by alcohol consumption, gall stones and various viruses and drugs. We present a case of a young female, who was recently diagnosed as COVID-19 and later on developed acute pancreatitis without any other risk factors.
Key Words: COVID-19, SARS-CoV-2, Acute pancreatitis.
INTRODUCTION
The ongoing pandemic of coronavirus disease-2019 (COVID-19) has spread across more than 200 countries and has caused approximately over one million deaths. Patients infected with COVID-19 virus disease commonly present with fever, headache, fatigue, cough and dyspnea.1 However, various cardiovascular, renal, thrombotic, neurological and gastrointestinal complications have been reported in association with COVID-19 in the literature. Gastrointestinal symptoms in COVID-19 patients commonly present as anorexia, vomiting, abdominal pain and diarrhea. Rarely, acute pancreatitis caused by SARS-CoV-2 has been reported in the literature.2,3 Herein, we present a rare complication of SARS-CoV-2 infection in a young female who developed acute pancreatitis on the second day of her admission with no other causative factor for acute pancreatitis, aside from SARS-CoV-2 infection.
CASE REPORT
A 30-year female with the insignificant past medical history came to the Emergency Department with a three-day history of dry cough, fever and fatigue. On examination, blood pressure was 110/70 mmHg, pulse was 105/min, respiratory rate 24 breaths/min, temperature 100˚F and oxygen saturation was 87% at room air.
Chest examination showed bilateral basal crepitations. She was admitted as a suspected case of COVID-19 and was started on oxygen inhalation (6 L/min), intravenous antibiotics including azithromycin (500 mg/day), ceftriaxone (75 mg/kg/day), dexamethasone (6 mg/day IV), and subcutaneous low molecular weight heparin (40 mg BD subcutaneously). Baseline investigations and a nasopharyngeal swab test for COVID-19 on quantitative reverse-transcriptase-polymerase-chain reaction (qRT-PCR) assay, were sent for analysis. Baseline investigations showed raised inflammatory markers and a high total leukocyte count (Table I).
Table I: Laboratory findings.
|
Test |
Result |
|
Hemoglobin |
11.1 g/dl |
|
Total leukocyte count |
14.9x109/l |
|
Red blood cells |
4.1 x1012/l |
|
Platelets |
433x109/l |
|
Prothrombin time |
12 seconds (12 seconds control) |
|
Activated partial thromboplastin time |
32 seconds (28 seconds control) |
|
D-dimers |
1240 ng/FEUmL (reference value: upto 500 ng/FEUmL) |
|
C-Reactive protein |
51 mg/dL (reference value <0.5 mg/dL) |
|
Lactate dehydrogenase (LDH) |
625 U/L |
|
Serum Ferritin level |
1120.3 µg/L |
|
Serum lipase |
626 U/l (normal range 8-78 U/l) |
|
Serum amylase |
820 U/l (normal range 25-125 U/L) |
|
Blood urea |
47 mg/dL |
|
Creatinine |
1 mg/dL |
|
Sodium |
141 mEq/L |
|
Potassium |
4.2 mEq/L |
The following day, she developed severe epigastric pain radiating to back associated with nausea and one episode of vomiting. Examination showed severe upper abdominal tenderness. Ultrasound abdomen was done, which showed only fatty liver and mild left-sided pleural effusion. There was no evidence of gall stones or any other biliary pathology. Serum amylase and lipase were sent for analysis which showed a high serum amylase of 820 U/l (normal range 25-125 U/L) and serum lipase of 626 U/l (normal range 8-78 U/l). X-ray chest demonstrated bilateral infiltrates (Figure 1).
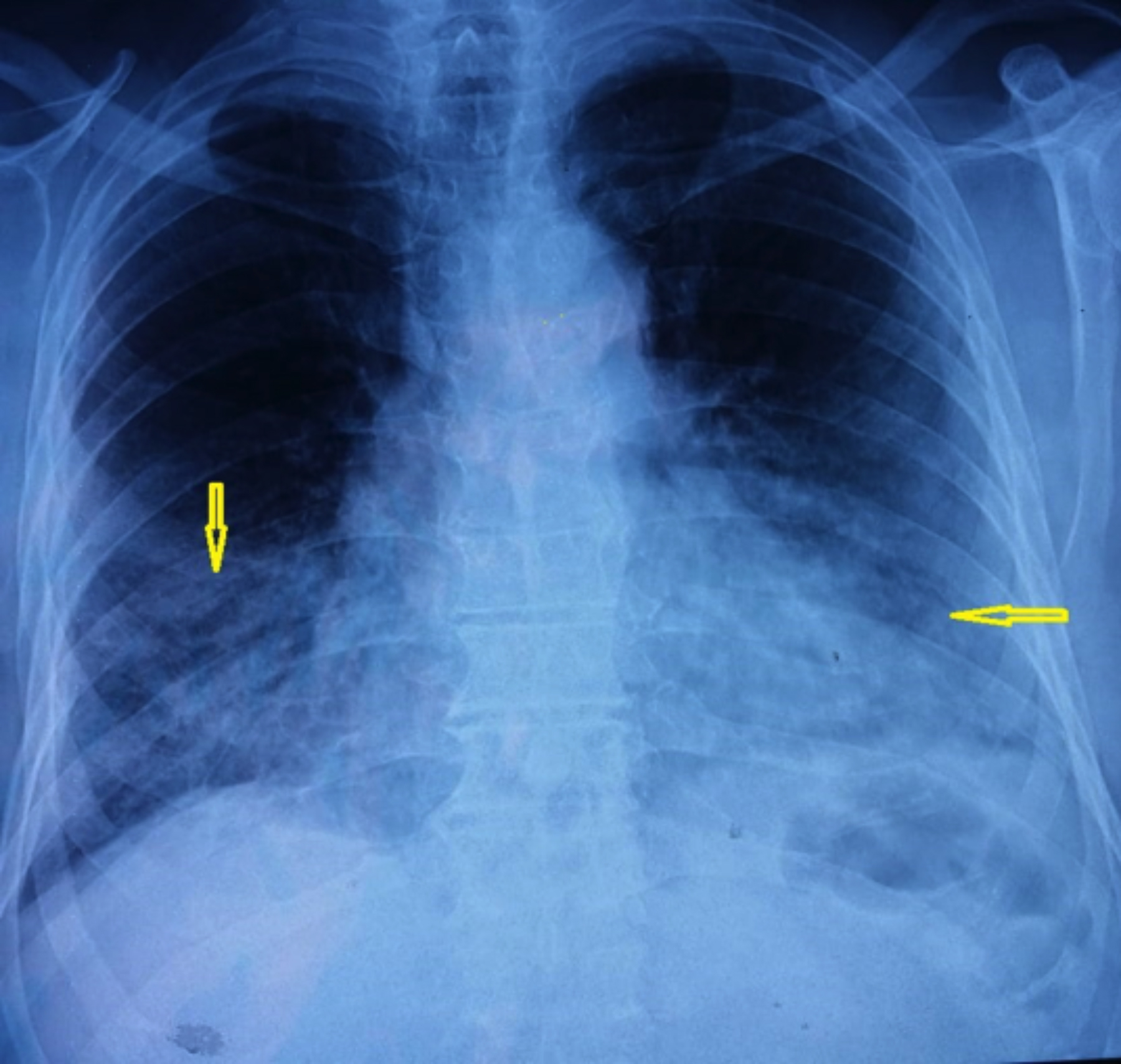 Figure 1: Chest X-ray showing bilateral interstitial infiltrates.
Figure 1: Chest X-ray showing bilateral interstitial infiltrates.
Table II: Laboratory findings.
|
Test |
Results |
|
Total cholesterol |
111 mg/dL |
|
Triglycerides |
119 mg/dL |
|
High-density lipids |
45 mg/dL |
|
Low-density lipids |
180 mg/dL |
|
S. Calcium |
8.3 mg/dL |
|
HBs antigen |
Negative |
|
Anti-HBs antibody |
Negative |
|
Anti-HBc antibody |
Negative |
|
Anti-HCV antibody |
Negative |
|
Anti-herpes virus 1 antibody ( IgM/IgG) |
Negative/ Negative |
|
Anti-herpes virus 2 antibody ( IgM/IgG) |
Negative/ Negative |
|
Anti-Coxsackie antibody ( IgM/IgG) |
Negative/ Negative |
|
Anti-CMV antibody ( IgM/IgG) |
Negative/ Negative |
|
ANA Screening |
Negative |
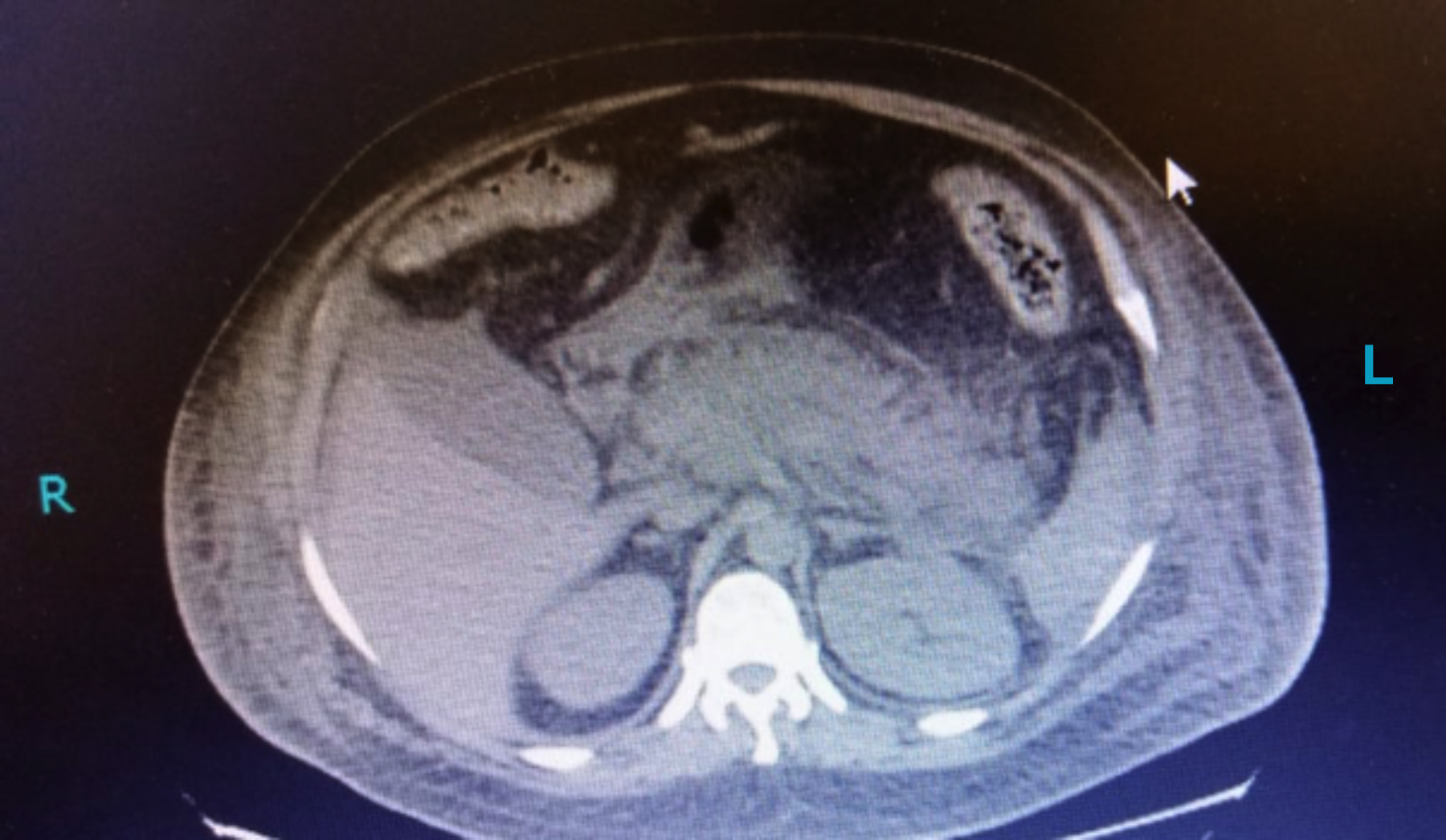 Figure 2: CT scan showing features of acute pancreatitis.
Figure 2: CT scan showing features of acute pancreatitis.
PaO2/FiO2 ratio was 250. A contrast-enhanced computed tomography scan was done, indicating an edematous, diffusely enlarged pancreas with ill-defined borders (Figure 2).
She was shifted to COVID-19 intensive care unit. A multidisciplinary team provided specialised care. The intravenous fluid was started along with non-steroidal anti-inflammatory drugs (NSAIDs) as needed, and she was kept nil by mouth (NBM) till further orders. On the second day of her admission, her PCR result came out positive for COVID-19. Serum lipid profile, serum calcium, anti-neutrophilic antibodies and virology were sent to determine the causative factor, the results of which were unremarkable (Table II).
She had no history of alcohol intake, smoking and drug abuse. She was diagnosed with acute pancreatitis secondary to SARS-CoV-2, as there were no other risk factors. She was started on soft diet on day 6 of her admission. Her condition improved significantly and was discharged on 7th day of admission in a stable condition with oxygen saturation of 95% without oxygen support.
DISCUSSION
The pathophysiology of acute pancreatitis involves several factors including enzyme activation, complement activation, pancreatic autodigestion, leukocyte activation, and pancreatic acinar cell necrosis and apoptosis. Coxsackievirus, hepatitis-A virus, measles virus, mumps virus and Epstein-Barr virus have been reported as the most common viral causes of pancreatitis in the literature.4 SARS-CoV-2 not only affects the respiratory system, but its association with the gastrointestinal symptoms has been documented in literature, and its RNA has been detected in the gastrointestinal tract.5 Theoretically, direct damage of pancreatic acinar cells by inflammation and oedema caused by the virus could be the possible reason for viral pancreatitis. Direct damage of pancreatic acinar cells by the virus, leading to leakage of intracellular enzymes and precipitating necrosis of the acinar cells could be one of the possibilities. SARS-CoV-2 enters into the host cells through angiotensin-converting enzyme 2 (ACE2) and these receptors have been detected in the gastrointestinal tract.6 Only a few cases of SARS-Cov-2-induced pancreatitis have been reported so far. Cheung et al. reported a case of recurrent acute pancreatitis in a 38-year healthy individual with no other contributing risk factor except SARS-CoV-2 infection.7 Similarly, Liu et al. revealed that out of 67 severe COVID-19 patients, 17% had increased levels of amylase and lipase. The authors also reported that in 7.46% of the COVID-19 patients, pancreatic injuries were evident on computed tomography scan.8 The typical presentation of acute pancreatitis in this case without any risk factors except SARS-CoV-2 infection, support this idea that this virus could be the cause of acute pancreatitis.
The physician should be vigilant enough about this possible complication because early intervention could decrease the mortality associated with this condition. During this ongoing pandemic, the physicians should always keep in mind such complication to prevent poor outcome.
PATIENT'S CONSENT:
Written informed consent for publication was obtained from the patient.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS' CONTRIBUTION:
MH: Conceived and designed the study.
MH, AWK: Responsible for data collection.
MH, AWK, SU, FS, SJK: Wrote the initial manuscript.
MH, SU, SJK: Critically revised the manuscript.
All authors have read the final manuscript.
REFERENCES
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020; 323(11):1061-9. doi:10.1001/jama.2020.1585.
- Kataria S, Sharif A, Rehman A, Ahmed Z, Hanan A. COVID-19 induced acute pancreatitis: A case report and literature review. Cureus 2020; 12(7):e9169. doi:10.7759/ cureus.9169.
- Meireles PA, Bessa F, Gaspar P, Parreira I, Silva VD, Mota C, et al. Acalculous acute pancreatitis in a COVID-19 patient. Eur J Case Rep Intern Med 2020; 7(6):001710. doi:10.12890/2020_001710.
- Kottanattu L, Lava SAG, Helbling R, Simonetti GD, Bianchetti MG, Milani GP. Pancreatitis and cholecystitis in primary acute symptomatic Epstein-Barr virus infection - Systematic review of the literature. J Clin Virol 2016; 82:51-5. doi:10.1016/j.jcv.2016.06.017.
- Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: Systematic review and meta-analysis. Gastroenterology 2020; 159(1):81-95. doi:10.1053/j.gastro.2020.03.065.
- Hadi A, Werge M, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, et al. Coronavirus disease-19 (COVID-19) associated with severe acute pancreatitis: Case report on three family members. Pancreatology 2020; 20(4):665-7. doi:10.1016/j.pan.2020.04.021.
- Cheung S, Delgado Fuentes A, Fetterman AD. Recurrent acute pancreatitis in a patient with COVID-19 infection. Am J Case Rep 2020; 21:e927076. doi:10.12659/AJCR.927076.
- Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol 2020; 18(9):2128-2130.e2. doi:10.1016/j.cgh.2020.04.040.