An Evaluation of the Effect of Preoperative Inflammation-based Factors on Survival in Gastric Cancer Patients
By Abdullah Durhan1, Abdullah Senlikci1, Koray Kosmaz1, Ender Erguder1, Umit Mercan2, Marlen Suleyman1Affiliations
doi: 10.29271/jcpsp.2021.03.282ABSTRACT
Objective: To investigate the predictive effect of preoperative inflammatory factors on overall survival (OS) in patients diagnosed with gastric adenocarcinoma (GAC).
Study Design: Observational study.
Place and Duration of Study: Department of General Surgery, Ankara Training and Research Hospital, between January 2011 and October 2020.
Methodology: A retrospective examination of 207 patients was made from the demographic, preoperative, and postoperative clinical pathology records of patients diagnosed with GAC. Demographic data, pathological tumor–node–metastasis (TNM) staging, preoperative inflammatory factors including neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR), and aspartate transaminase (AST)-alanine aminotransaminase (ALT) ratio, red cell distribution width (RDW), and hypoalbuminemia were statistically analysed in respect of the effect on OS.
Results: Overall survival was determined to be significantly shorter in patients with age >65 years (p = 0.001), advanced TNM stage (p <0.001), tumor size >4.7 cm (p = 0.007), AST-ALT ratio >1.21 (p = 0.017), and hypoalbuminemia (<35 g / L) (p = 0.018). In Cox regression analysis for all factors affecting OS, age >65 years (p = 0.002) and TNM stage 1B (p = 0.004) and 2A (p = 0.039) were determined as independent predictors of survival. The values of NLR, PLR, and RDW were not statistically significant between the groups with and without mortality (p=0.066, p=0.283, p=0.501, respectively).
Conclusion: Inflammation-based factors including AST-ALT ratio and albumin can help assess prognosis in patients with gastric cancer in standard clinical preoperative tests.
Key Words: Gastric cancer, Inflammation-based factors, Aspartate transaminase-alanine aminotransaminase ratio, Neutrophil-lymphocyte ratio, Platelet-lymphocyte ratio, Albumin, Tumor–node-metastasis staging, Overall survival.
INTRODUCTION
Gastric cancer (GC) is the 5th most prevalent form of cancer in the world and ranks 3rd among cancer-related causes of death after lung cancer and colorectal cancer. It is a form of cancer with a poor prognosis, despite the identification of decreasing incidence and mortality rates over the years and improvements in treatment methods.1 The difference in overall survival (OS) may depend on the stage of disease at diagnosis and the level of surgery and lymph node dissection performed in patients at the same stage.2
The tumor-node-metastasis (TNM) staging mechanism is known to be the key factor in the prediction of gastric cancer prognosis.3 However, the OS of gastric adenocarcinoma (GAC) patients, at the same level of the disease, is independent of surgery as it could be entirely different. It is understood that the efficacy of curative resection is as important as certain patient-related factors, such as preoperative dietary status and comorbid conditions, which have a pathological effect on postoperative outcomes and OS.4
Clinical prognosis in cancer patients is related to the characteristics of the tumor as much as the patient's response to the tumor. Especially in recent years, it has been proven that systemic inflammatory response has a significant role in tumor growth and invasion development. The systemic inflammatory response is important in carcinogenesis and tumor progression; and is associated with short postoperative OS in patients with various types of cancer.5
Numerous studies have been performed on the neutrophil-lymphocyte ratio (NLR) and the platelet-lymphocyte ratio (PLR) used as inflammatory indicators in preoperative staging and OS in various forms of cancer.6,7 Aspartate aminotransaminase (AST) and alanine aminotransaminase (ALT) are liver enzymes that are widely used in clinical laboratory tests. The AST-ALT ratio, also known as the De Ritis ratio, was originally described by De Ritis in 1957.8 While De Ritis was used for the first time in the evaluation of viral hepatitis and some chronic liver diseases, recent studies have used it as an independent prognosis factor to identify the stage of the disease and OS in some malignancies.9,10 There are some drawbacks to the use of TNM classification alone to predict OS in GAC patients.4 It is also clinically essential to recognise other prognostic factors linked to GAC. Serum markers are simple to calculate, replicable, and low-cost tests, which are helpful in the diagnosis, measurement of OS rates, and follow-up of patients after surgery, and in the closer supervision of patients in a higher risk group.
The aim of this study was to determine the effect of markers such as albumin, red cell distribution width (RDW), NLR, PLR, and the AST-ALT ratio, which are preoperatively evaluated serum markers on survival in patients with GAC.
METHODOLOGY
This study was approved by the Ethics Committee of Ankara Training and Research Hospital (No. 2020/20:475, dated 26.11.2020). A retrospective analysis was made from the data of patients, who presented at the General Surgery Clinic between January 2011 and October 2020; and were operated with a diagnosis of GAC. Data were collected from the Hospital information database by four surgeons (AD, AŞ, KK, MS). Mortality information was accessed from the National Death Notification System. Patients were excluded from the study, if all data were not available, if they had a diagnosis of a gastric malignancy other than GAC (neuroendocrine tumor, gastrointestinal stromal tumor, lymphoma), had recurrence disease, a diagnosis of synchronous or metachronous malignancy had received preoperative chemotherapy and/or radiotherapy, if emergency surgery was performed for bleeding, obstruction or perforation, had a hematological disease, or chronic liver disease.
A total of 207 patients were included in the study. Patient clinicopathological analyses, such as age, gender, tumor size, tumor differentiation, tumor localisation, lymph node involvement, the existence of distant metastases, surgical margin status, the form of procedure, whole blood count outcomes, albumin, carcinoembryonic antigen (CEA), and carbohydrate antigen (CA19-9) data, were accessed retrospectively from the database. The ratios of NLR, PLR, and AST-ALT were electronically calculated, using a basic method of proportion. Tumor staging was applied according to the AJCC 8th edition of the TNM staging system released in 2017.11
Data were analysed statistically using IBM ® SPSS version. 23.0 software. Variables were expressed as mean ± standard deviation (SD) and median (IQR: 25th percentile-75th percentile) values or as number (n) and percentage (%). Histogram graphics and the Kolmogrov-Smirnov test were used to evaluate the conformity to the normal distribution of quantitative variables. The Chi-square or the Likelihood ratio were used as appropriate to determine the associations between categorical variables. The Student’s t-test and the Mann-Whitney U-test were used for numerical variables for comparison between two independent groups. The optimum cut-off values of the NLR, PLR, and AST-ALT ratios were calculated using receiver operating characteristic curve (ROC) analysis. Log rank test and Kaplan-Meier survive analysis were used to compare the effects of clinicopathological and prognostic variables on OS. The Cox proportional regression model with the backward elimination stepwise method was used in the multivariate analysis of overall survival. A value of p <0.05 was accepted as statistically significant.
RESULTS
The study group included 207 patients with a mean age of 67.57 ± 13.43 years. One hundred and fifty (72.5%) patients were males and 57 (27.5%) patients were females. Mortality was found in 133 (64.3%) patients. When the patients were divided into two groups as those with and without mortality, the patients who developed mortality were statistically significantly of older age (70.75 ± 12.81 years vs. 61.85 ± 12.68 years, p <0.001), had more advanced stage tumors (p <0.001), larger tumors (6.21 ± 2.63 vs. 4.84 ± 2.74, p = 0.001), and a higher surgical margin positivity rate (p = 0.002). There was no significant difference between the groups in terms of tumor differentiation (p = 0.132). When the groups were compared in terms of preoperative laboratory values and inflammatory scores, AST-ALT ratio [1.40 (1.11-1.86) vs. 1.21(1.04-1.51) p = 0.004], CEA level [2.40 (1.38-3.41) vs. 1.84 (1.09-3.13), p = 0.018] and CA 19-9 level [ 25.30 (13.67-42.97) vs. 12.95 (6.78-24.52), p <0.001] were found to be statistically significantly higher in the group that developed mortality, while the albumin level [38.00 (32.50-42.00) vs. 40.00 (35.67-43.70), p = 0.004] and lymphocyte count [1.60 (1.30-2.24) vs. 1.93 (1.50-2.53), p = 0.017] were lower. RDW was not associated with mortality (p=0.501). The values of NLR and PLR were found higher in the group with mortality. But, no statistically significant findings were obtained (p values for NLR and PLR = 0.066 and 0.283, respectively). The comparisons of demographic and clinicopathological variables between the two groups with and without mortality are summarised in Table I.
The mean survival of the whole study population was found to be 50.39 ± 4.01 months (95% CI: 42.53-58.25). The cumulative survival rate was 64.1% at 1 year, 41.1% at 3 years, 32.9% at 5 years, and 26.5% at 10 years (Figure I).
When ROC curve analysis was applied for AST-ALT ratio and tumor size, a significant difference was determined between the groups, with the cut-off values for mortality development of 1.21 (65.4% sensitivity, 50% specificity), (AUC: 0.621, 95% CI: 0.544 -0.698) and 4.7 cm (75.9% sensitivity, 50% specificity) (AUC: 0.656, 95% CI: 0.574 - 0.738), respectively.
Table I: Comparison of demographic and clinicopathological variables between the two groups with and without mortality.|
Variables |
Total (n=207) |
Mortality (-) (n=74) |
Mortality (+) (n=133) |
p-value |
|
Age (years) |
67.57±13.43 |
61.85±12.68 |
70.75±12.81 |
<0.001 |
|
Gender (male) n (%) |
150(72.5%) |
50(67.6%) |
100(75.2%) |
0.239 |
|
Tumor Localisation n (%) Cardia Corpus Antrum linitis plastica |
36(17.4%) 75(36.2%) 92(44.4%) 4(1.9%) |
8(10.8%) 32(43.2%) 34(45.9%) 0(0%) |
28(21.1%) 43(32.3%) 58(43.6%) 4(3%) |
0.042 |
|
Operation n (%) Total Subtotal Palliative surgery Laparoscopic Total Laparoscopic Subtotal |
89(43%) 86(41.5%) 13(6.3%) 4(1.9%) 15(7.2%) |
26(35.1%) 36(48.6%) 1(1.4%) 3(4.1%) 8(10.8%) |
63(47.4%) 50(37.6%) 12(9%) 1(0.8%) 7(5.3%) |
0.010 |
|
T Stage n (%) T1 T2 T3 T4 |
20(9.7%) 47(22.7%) 103(49.8%) 37(17.9%) |
16(21.6%) 23(31.1%) 25(33.8%) 10(13.5%) |
4(3%) 24(18%) 78(58.6%) 27(20.3%) |
<0.001 |
|
N Stage n (%) N0 N1 N2 N3 Nx |
63(30.4%) 31(15%) 41(19.8%) 59(28.5%) 13(6.3%) |
34(45.9%) 12(16.2%) 12(16.2%) 15(20.3%) 1(1.4%) |
29(21.8%) 19(14.3%) 29(21.8%) 44(33.1%) 12(9%) |
0.002 |
|
M Stage n (%) M0 M1 |
187(90.3%) 20(9.6%) |
72(97.3%) 2(2.7%) |
115(86.5%) 18(13.5%) |
0.021 |
|
TNM Stage n (%) 1A 1B 2A 2B 3A 3B 3C 4 |
20(9.7%) 24(11.6%) 30(14.5%) 26(12.6%) 31(15%) 47(22.7%) 11(5.3%) 18(8.7%) |
15(20.3%) 14(18.9%) 11(14.9%) 10(13.5%) 7(9.5%) 14(18.9%) 1(1.4%) 2(2.7%) |
5(3.8%) 10(7.5%) 19(14.3%) 16(12%) 24(18%) 33(24.8%) 10(7.5%) 16(12%) |
<0.001 |
|
Tumor Size (cm) |
5.72±2.74 |
4.84±2.74 |
6.21±2.63 |
0.001 |
|
Surgical margin status n (%) Negative Positive Insignificant |
187(90.3%) 7(3.4%) 13(6.3%) |
73(98.6%) 0(0%) 1(1.4%) |
114(85.7%) 7(5.3%) 12(9%) |
0.002 |
|
Tumor Differentiation n (%) good moderate poor |
43(20.8%) 49(23.7%) 115(55.6%) |
21(28.4%) 16(21.6%) 37(50%) |
22(16.5%) 33(24.8%) 78(58.6%) |
0.132 |
|
AST (U/L) |
20.44±12.08 |
22.36±17.46 |
19.38±7.48 |
0.164 |
|
ALT (U/L) |
13.00(10.00-19.00) |
14.50(11.00-21.00) |
13.00(8.50-19.00) |
0.026 |
|
AST/ALT ratio |
1.37(1.07-1.70) |
1.21(1.04-1.51) |
1.40(1.11-1.86) |
0.004 |
|
Albumin(g/L) |
38.80(33.50-42.00) |
40.00(35.67-43.70) |
38.00(32.50-42.00) |
0.004 |
|
CEA (ng/ml) |
2.22(1.28-3.31) |
1.84(1.09-3.13) |
2.40(1.38-3.41) |
0.018 |
|
CA 19-9 (U/ml) |
19.42(9.40-37.30) |
12.95(6.78-24.52) |
25.30(13.67-42.97) |
<0.001 |
|
Neutrophil (10⁹/L) |
5.08±2.19 |
5.02±2.12 |
5.12±2.24 |
0.735 |
|
Lymphocyte(10⁹/L) |
1.70(1.34-2.30) |
1.93(1.50-2.53) |
1.60(1.30-2.24) |
0.017 |
|
RDW (%) |
15.20(13.80-17.20) |
15.10(13.30-17.63) |
15.40(13.90-17.00) |
0.501 |
|
Platelet (10⁹/L) |
279.94±100.04 |
295.03±107.06 |
271.55±95.29 |
0.106 |
|
NLR |
2.56(1.79-3.50) |
2.39(1.66-3.22) |
2.65(1.93-3.73) |
0.066 |
|
PLR |
147.83(103.91-211.25) |
138.77(98.98-204.51) |
149.23(110.00-215.36) |
0.283 |
|
TNM: Tumor-node-metastasis, AST: Aspartate aminotransaminase, ALT: Alanine aminotransaminase, CEA: Carcinoembryonic antigen, CA 19-9: Carbohydrate antigen, RDW: Red cell distribution width, NLR: Neutrophil-lymphocyte ratio, PLR: Platelet-lymphocyte ratio, Nx: No diagnostic tools were used to evaluate the status of lymph nodes. |
||||
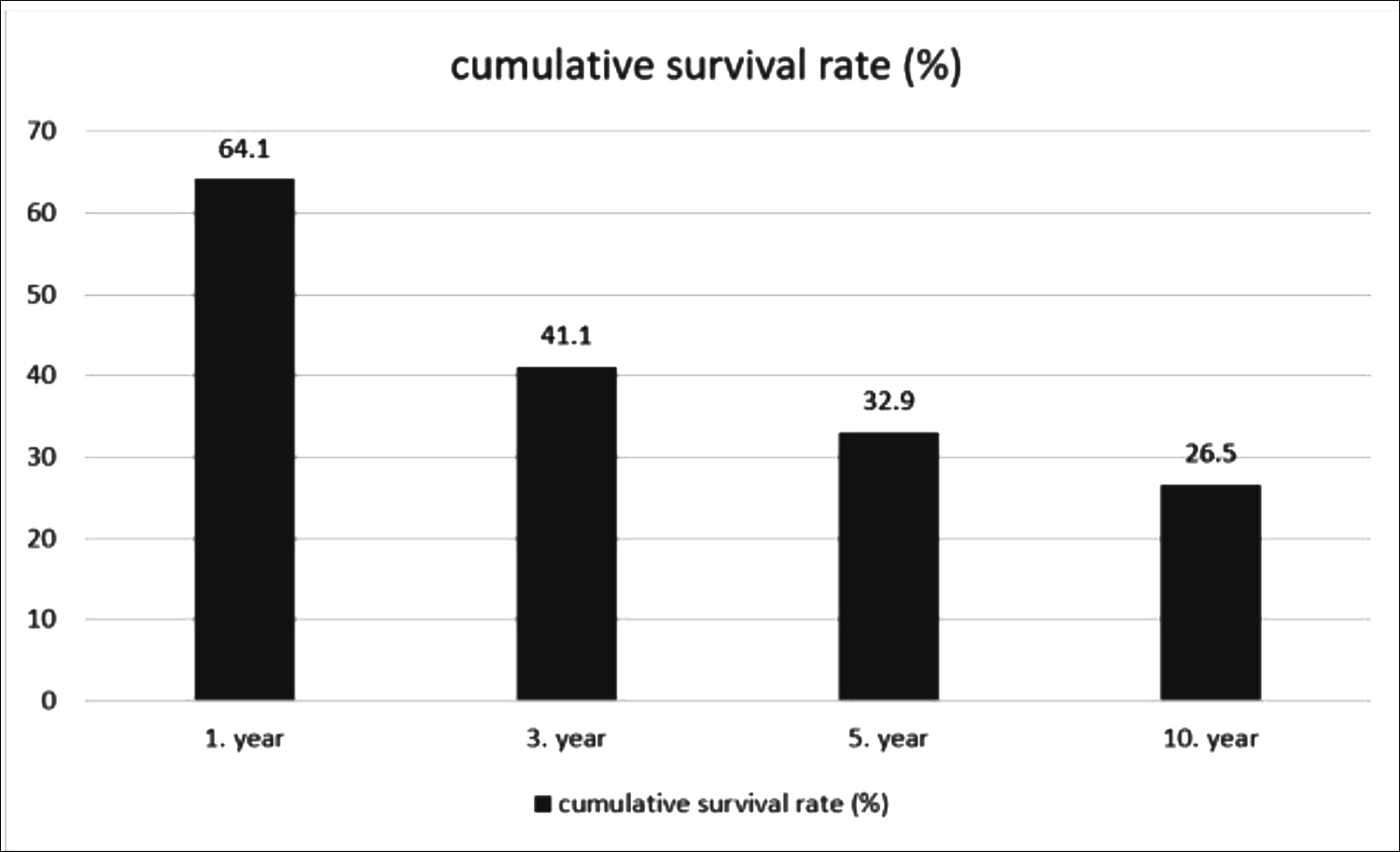 Figure I: Cumulative survival rate of patients by years.
Figure I: Cumulative survival rate of patients by years.
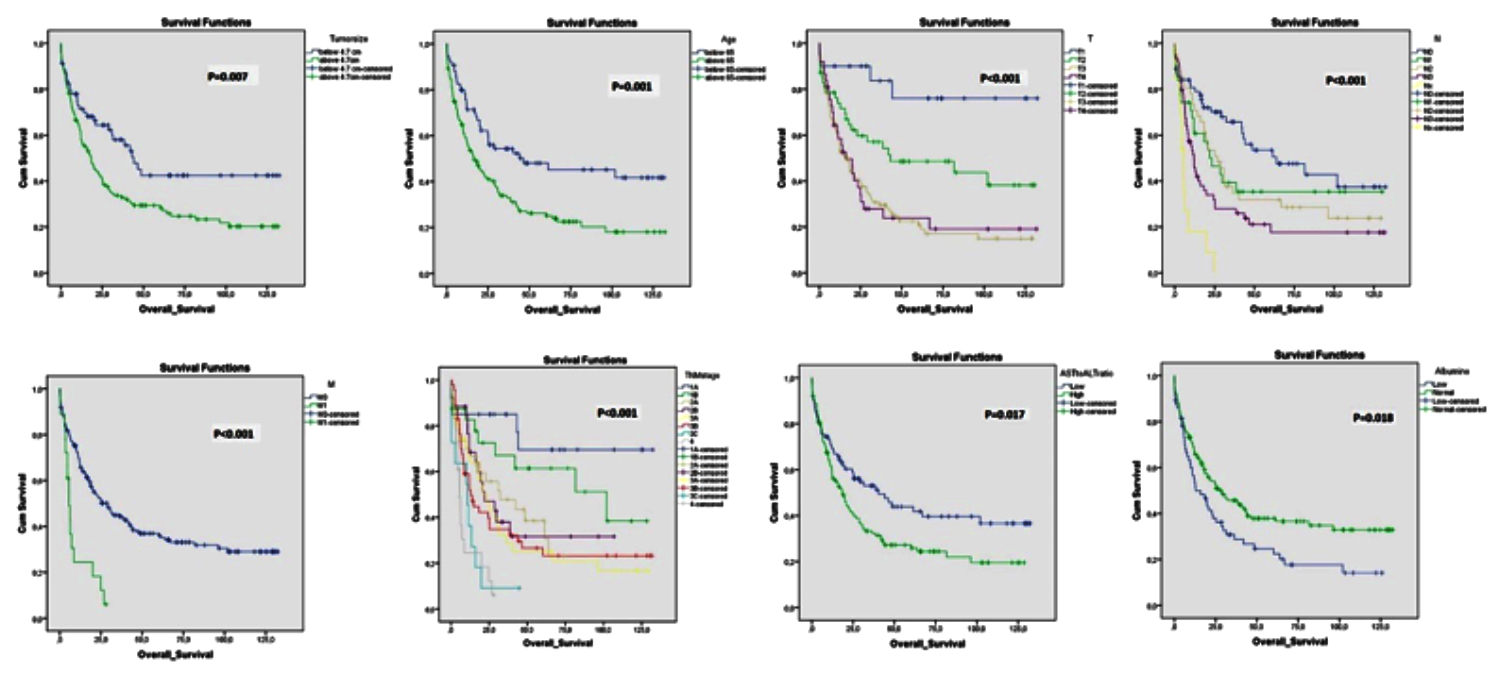
Figure 2: Kaplan Meier survival graphs showing the effects of demographic and clinicopathological variables on survival.
The survival time was determined to be statistically significantly shorter for patients aged >65 years (p=0.001), with advanced TNM stage (p<0.001), advanced T stage (p<0.001), advanced N stage (p<0.001), M1 disease (p<0.001), tumor size >4.7 cm (p =0.007), AST / ALT ratio >1.21 (p = 0.017), and hypoalbuminemia (<35) (p = 0.018). Kaplan Meier survival charts showing the effects of demographic and clinicopathological variables on survival are shown in Figure 2.
In the Cox regression analysis for all factors affecting survival, age >65 years (HR: 0.52, 95% CI 0.35-0.78, p = 0.002) and TNM stage 1B (HR: 0.12, % 95 C.I: 0.03-0.52, p = 0.004) and 2A (HR: 0.27, % 95C.I: 0.08-0.94, p= 0.039) were determined to be independent predictors of survival.
DISCUSSION
Although early-stage GC patients have long survival rates following curative surgical surgery and/or systematic adjuvant therapy, the findings of late-stage clinical studies and the fact that cancer screening services such as for other colon and breast cancer, are not universal, indicate that patients with GC are diagnosed at late-stage. Moreover, recurrence and metastases appear to be the key causes that can affect recovery in most cases, even after curative resection.12
The TNM staging mechanism is now recognised as the key predictor for determining the prognosis of gastric cancer.3,11 In this study, survival was observed to be lower in patients with advanced TNM stage (p<0.001) in GAC. However, certain patients at the same TNM level can have a different prognosis.13 It is necessary to predict the treatment response of patients with GC and prognostic factors other than TNM, which will predict OS to enable the close follow-up of high-risk patients.
Cancer and immunology have become increasingly relevant. Immunologists have suggested that the immune response has been weakened and the response to the tumor is diminished in cancer patients.14 Various studies have been conducted on different forms of the inflammatory response in cancers.6,7 NLR and PLR, which can be obtained from peripheral full blood count, are routine clinical markers for systemic inflammatory response. Pre-treatment with peripheral neutrophils and platelets and reduced lymphocyte counts have been found to have a detrimental impact on the survival of cancer patients. Platelet activation regulates tumor growth and spread through neoangiogenesis, extracellular matrix degradation, adhesion molecules, and growth factor expression. Neutrophils causing tumor growth are the primary source of angiogenesis and growth factors, and lymphocytes provide immunotoxic cell death and cytotoxin production, which prevent the spread of tumor cells.15,16 Various findings have been observed in NLR and PLR trials in patients with GC. Although, generally meaningful findings have been observed for high NLR, and low survival for PLR, other studies have not obtained this outcome; and the cut-off values for these OS scores have generally been different in each sample.17,18 In the current study, NLR (p = 0.066) and PLR (p = 0.283) were found to be correlated with mortality, but no statistically significant findings were obtained.
Preoperative nutritional status is one of the variables related to cancer in the hospital. Serum albumin is formed in the liver and is the most abundant plasma protein in the body. Serum albumin is the conventional standard component used to determine the nutritional health of a patient. Recent studies have demonstrated that albumin is a dietary or inflammatory predictor. In patients who have been fasting for a long time, the albumin value does not decline immediately, but declines shortly after surgical discomfort, suggesting that albumin is a harmful acute inflammatory protein rather than a dietary predictor.19 Preoperative albumin value tests are typically studies of postoperative complication risk. Current literature studies have reported that postoperative complications and overall survival are associated with albumin and various inflammatory markers, such as the CRP/albumin ratio20 and the prognostic nutrition index (PNI), which are easily calculated from albumin values and lymphocyte counts.21 In the current study, statistically significant findings were obtained between preoperative hypoalbuminemia and OS (p = 0.018). Patients with GC who are malnourished should be hospitalised preoperatively, if possible, and dietary care should be offered. Generally, these cachectic patients should be examined more comprehensively in terms of being at the early stage of the condition, and the patients receiving therapy should be monitored closely.
The effect of the AST-ALT ratio on cancer prognosis has been documented in several studies. These studies are often related to non-GC malignancies; and in particular, the majority of studies of the urinary system have drawn attention to this.22 There are very few studies in the literature on GC and the AST-ALT ratio, although there are several theories concerning the increasing use of the AST-ALT ratio in cancer patients. Cancer cells rather than normal cells promote aerobic glycolysis, in which AST plays a significant role. This pathological state contributes to the triggering of more AST than ALT in rapidly developing cancer tissue.23 AST is found mainly in the mitochondria of the cells, while ALT is found only in the cytoplasm of the hepatocytes. In cases involving tissue injury, mitochondrial DNA is weakened due to the release of reactive oxygen species, which can cause significant tissue damage and lead to the release of more mitochondrial enzymes.24 Therefore, the high AST-ALT ratio could be an OS-related prognosis predictor in patients with various types of cancer. Although the cut-off value for the preoperative AST-ALT ratio was 1.21 in the current study analysis, different parameters have been used, yielding different cut-off values such as 1.1 and 1.35 in other studies.10,22 In the current study, patients with an AST/ALT ratio of >1.21 were determined to have a statistically significant shorter survival time (p = 0.017).
In this study, age (>65) and increased tumor diameter (>4.7 cm) were other findings linked to poor survival, while positive surgical margin and elevated CEA and CA 19-9 tumor markers were statistically significant and consistent with the findings of mortality in literature. From these significant findings, it can be strongly recommended that patients with these results should be studied in more depth in terms of late-stage disease and should be more closely tracked in terms of survival.
There were some limitations to this study. Primarily, it was a single-centre observational study with a limited sample size of 207 patients. Second, due to lack of available data, it was not possible to determine medical problems that could impact the immune system of the patients. So, there is a need for further multicentre, prospective randomised trials with greater sample sizes to verify these results.
While OS is recognised as the typical predictor for cancer prognosis, data on disease-free survival were absent from this study.25
CONCLUSION
The AST-ALT ratio and albumin value tend to be useful in predicting prognosis in patients with gastric cancer in routine clinical preoperative assessment, as serum markers are a simple, easy, and non-invasive examination. However, more rigorous clinical trials should be performed to confirm the prognostic function of preoperative inflammation-based factors patients with gastric malignancy.
ETHICAL APPROVAL:
Ankara Training and Research Hospital, Clinical Research Ethic Committee approved the study.
PATIENTS' CONSENT:
Because this study was retrospective, the patients' consents were waived.
CONFLICT OF INTEREST:
The authors have no conflict of interest to declare.
AUTHORS’ CONTRIBUTION:
AD: Led and conceived the project, and authored the manuscript.
AS: Data collection, compiling and discussion.
KK: Contributed to design articles, collected and analysed data.
EE: Contributed to collect and analyse data.
UM: Helped perform the analyses and statistics.
MS: Collected and analysed data, literature review and discussion.
REFERENCES
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68(6): 394-424. doi: 10.3322/caac.21492.
- Markar SR, Karthikesalingam A, Jackson D, Hanna GB. Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: comparison between West and East. Ann Surg Oncol 2013; 20(7):2328-38. doi: 10.1245/s10434-012-2862-9.
- Fujitani K, Kurokawa Y, Takeno A, Endoh S, Ohmori T, Fujita J, et al. Time to initiation or duration of S-1 adjuvant chemotherapy; which really impacts on survival in stage II and III gastric cancer? Gastric Cancer 2018; 21(3):446-52. doi: 10.1007/s10120-017-0767-9.
- Choi WJ, Kim J. Nutritional care of gastric cancer patients with clinical outcomes and complications: A review. Clin Nutr Res 2016; 5(2):65-78. doi: 10.7762/cnr.2016.5.2.65.
- Roxburgh CS, McMillan DC. Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 2010; 6(1):149-63. doi: 10.2217/fon.09.136.
- Sugiura T, Uesaka K, Kanemoto H, Mizuno T, Okamura Y. Elevated preoperative neutrophil-to-lymphocyte ratio as a predictor of survival after gastroenterostomy in patients with advanced pancreatic adenocarcinoma. Ann Surg Oncol 2013; 20(13):4330-7. doi: 10.1245/s10434-013- 3227-8.
- Jia J, Zheng X, Chen Y, Wang L, Lin L, Ye X, et al. Stage-dependent changes of preoperative neutrophil to lymphocyte ratio and platelet to lymphocyte ratio in colorectal cancer. Tumour Biol 2015; 36(12):9319-25. doi: 10.1007/s13277- 015-3667-9.
- De Ritis F, Coltorti M, Giusti G. An enzymic test for the diagnosis of viral hepatitis; the transaminase serum activities. Clin Chim Acta 1957; 2(1):70-4. doi: 10.1016/ 0009-8981 (57)90027-x
- Thornburg JM, Nelson KK, Clem BF, Lane AN, Arumugam S, Simmons A, et al. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res 2008; 10(5):R84.
- Lee H, Lee SE, Byun SS, Kim HH, Kwak C, Hong SK. De Ritis ratio (aspartate transaminase/alanine transaminase ratio) as a significant prognostic factor after surgical treatment in patients with clear-cell localised renal cell carcinoma: A propensity score-matched study. BJU Int 2017; 119(2): 261-7. doi: 10.1111/bju.13545
- In H, Solsky I, Palis B, Langdon-Embry M, Ajani J, Sano T. Validation of the 8th edition of the ajcc tnm staging system for gastric cancer using the national cancer database. Ann Surg Oncol 2017; 24(12):3683-91. doi: 10.1245/s10434- 017-6078-x.
- Rohatgi PR, Yao JC, Hess K, Schnirer I, Rashid A, Mansfield PF, et al. Outcome of gastric cancer patients after successful gastrectomy: Influence of the type of recurrence and histology on survival. Cancer 2006; 107(11):2576-80. doi: 10.1002/cncr.22317.
- Yoon HM, Ryu KW, Nam BH, Cho SJ, Park SR, Lee JY, et al. Is the new seventh AJCC/UICC staging system appropriate for patients with gastric cancer? J Am Coll Surg 2012; 214(1):88-96. doi: 10.1016/j.jamcollsurg.2011.09.018.
- Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, Watkins SC, et al. The tumor microenvironment represses t cell mitochondrial biogenesis to drive intratumoral T Cell metabolic insufficiency and dysfunction. Immunity 2016; 45(3):701-3. doi: 10.1016/j.immuni. 2016.07.009.
- Sun X, Liu X, Liu J, Chen S, Xu D, Li W, et al. Preoperative neutrophil-to-lymphocyte ratio plus platelet-to-lymphocyte ratio in predicting survival for patients with stage I-II gastric cancer. Chin J Cancer 2016; 35(1):57. doi: 10.1186/ s40880-016-0122-2
- Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer 2006; 42(6):768-78. doi: 10.1016/j.ejca.2006.01.006.
- Wang DS, Ren C, Qiu MZ, Luo HY, Wang ZQ, Zhang DS, et al. Comparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancer. Tumour Biol 2012; 33(3):749-56. doi: 10.1007/s13277-011-0285-z.
- Jiang N, Deng JY, Liu Y, Ke B, Liu HG, Liang H. The role of preoperative neutrophil-lymphocyte and platelet-lymphocyte ratio in patients after radical resection for gastric cancer. Biomarkers 2014; 19(6):444-51. doi: 10.3109/ 1354750X.2014.926567.
- Nazha B, Moussaly E, Zaarour M, Weerasinghe C, Azab B. Hypoalbuminemia in colorectal cancer prognosis: Nutritional marker or inflammatory surrogate? World J Gastrointest Surg. 2015; 7(12):370-7. doi: 10.4240/wjgs. v7.i12. 370.
- Liu Z, Jin K, Guo M, Long J, Liu L, Liu C, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation-based score in pancreatic cancer. Ann Surg Oncol 2017; 24(2):561-8. doi: 10.1245/s10434-016-5579-3.
- Saito H, Kono Y, Murakami Y, Kuroda H, Matsunaga T, Fukumoto Y, et al. Influence of prognostic nutritional index and tumor markers on survival in gastric cancer surgery patients. Langenbecks Arch Surg 2017; 402(3):501-7.doi: 10.1007/s00423-017-1572-y.
- Mori K, Janisch F, Mostafaei H, Kimura S, Lysenko I, Karakiewicz PI, et al. Prognostic role of preoperative De Ritis ratio in upper tract urothelial carcinoma treated with nephroureterectomy. Urol Oncol 2020; 38(6):601 e17- e24. doi: 10.1016/j.urolonc.2020.02.008.
- Dang CV. Links between metabolism and cancer. Genes & development 2012; 26(9):877-90. doi: 10.1101/gad. 189365.112.
- Zheng J, Seier K, Gonen M, Balachandran VP, Kingham TP, D'Angelica MI, et al. Utility of serum inflammatory markers for predicting microvascular invasion and survival for patients with hepatocellular carcinoma. Ann Surg Oncol 2017; 24(12):3706-14. doi: 10.1245/s10434-017-6060-7.
- Kim Y, Spolverato G, Ejaz A, Squires MH, Poultsides G, Fields RC, et al. A nomogram to predict overall survival and disease-free survival after curative resection of gastric adenocarcinoma. Ann Surg Oncol 2015; 22(6): 1828-35. doi: 10.1245/s10434-014-4230-4.