Diagnostic Value of Serum Gastrin and Epidermal Growth Factor to the Gastric Ulcer Complicated with Upper Gastrointestinal Hemorrhage
By Ying Li, Yuping SongAffiliations
doi: 10.29271/jcpsp.2020.12.1269ABSTRACT
Objective: To explore the predictive value of serum gastrin (GAS), epidermal growth factor (EGF) levels in gastric ulcer complicated with acute upper gastrointestinal bleeding.
Study Design: A descriptive study.
Place and Duration of Study: Department of Emergency, Beijing Jiangong Hospital, China, from January 2019 to June 2020.
Methodology: One hundred and twenty-five patients with gastric ulcer and acute upper gastrointestinal bleeding were selected as Group A. One hundred and twenty-five patients with gastric ulcer and no upper gastrointestinal bleeding were selected as Group B. Logistic regression analysis was used to analyse the risk factors of gastric ulcer complicated with acute upper gastrointestinal bleeding. The value of serum GAS, EGF in early diagnosis of gastric ulcer with upper gastrointestinal bleeding was evaluated by receiver operating characteristic (ROC) curve.
Results: Univariate analysis showed statistically significant differences between Group A and Group B in taking non-steroidal anti-inflammatory drugs (NSAIDs), helicobacter pylori (Hp) infection, serum GAS and EGF (all p <0.001). Logistic regression analysis showed that raised serum GAS and serum EGF were independent risk factors for gastric ulcer and upper gastrointestinal bleeding (both p <0.001). The ROC area of serum EGF to predict gastric ulcer and acute upper gastrointestinal bleeding was 0.810 (95% CI: 0.753-0.867, p <0.001), greater than ROC area of serum GAS. At serum EGF of ≤109.95 pg/mL, had the 84.8%, sensitivity to predict gastric ulcer and acute upper gastrointestinal bleeding with specificity of 68.8%.
Conclusion: The predictive value of serum GAS and EGF is high for gastric ulcer complicated with acute upper gastrointestinal bleeding; the predictive value of serum EGF is greater than that of serum GAS.
Key Words: Gastric ulcer, Acute upper gastrointestinal bleeding, Serum, Gastrin (GAS), Epidermal growth factor (EGF), Logistic regression, Receiver operating characteristic (ROC) curve.
INTRODUCTION
Gastric ulcer mainly refers to tissue damage exceeding mucosal muscular layer, caused by gastric mucosa digestion by gastric digestive juice itself. It is an inflammatory necrotic lesion that occurs between the cardia and the pylorus.1 Peptic ulcer is a common cause of upper gastrointestinal hemorrhage.2 Acute upper gastrointestinal bleeding is a common complication of gastric ulcer. Most of the times, the bleeding volume is 300-500 mL. When the bleeding volume is more than 800 mL, systemic shock and even life-threatening may occur.3
Gastroscopy, also known as upper gastrointestinal endoscopy, is currently the preferred examination method for the etiology and location of upper gastrointestinal hemorrhage in diagnosis. An examination performed within 24-48 hours after hemorrhage is called an emergency gastroscopy. Upper gastrointestinal hemorrhage can be diagnosed by gastroscopy.4 Early gastroscopy and accurate diagnosis are the key to reduce mortality. Compared with endoscopy, blood examination has the advantages of low cost and low risk.5
Gastrin (GAS) is a peptide hormone synthesised and secreted by gastric antral G cells. After release, it acts on gastric parietal cells to stimulate the secretion of hydrochloric acid through improving the blood circulation, which plays an important role in regulating digestive function.6 One study has confirmed that serum GAS level in patients with gastric ulcer has increased.7
Epidermal growth factor (EGF) is a kind of endogenous protective substance, which can promote the proliferation, differentiation and migration of epithelial cells. EGF has the repairing and protecting function to gastric mucosa damage.8 A study has shown that serum EGF is involved in the occurrence and healing of gastric ulcers.9
At present, the application value of serum GAS and EGF in acute upper gastrointestinal bleeding, complicated with gastric ulcer, is not yet clear. In view of this, the purpose of this study was to explore the predictive value of serum GAS and EGF levels in gastric ulcer complicated with acute upper gastrointestinal bleeding.
METHODOLOGY
This descriptive study was approved by The Ethics Committee of Beijing Jiangong Hospital, China. A total of 125 patients with gastric ulcer and acute upper gastrointestinal bleeding admitted to the hospital from January 2019 to June 2020, were selected as Group A. One hundred and twenty-five patients with gastric ulcer and no upper gastrointestinal bleeding admitted to the hospital during the same period, were selected as Group B.
Inclusion criteria were that patients with gastric ulcer diagnosed by gastroscopy, without effective treatment before admission, complete medical history information, voluntary acceptance of this trial. Exclusion criteria were that patients aged >80 or <18 years; diagnosed with duodenal ulcer, pyloric duct ulcer and compound ulcer by gastroscopy; received peptic endoscopic hemostasis treatment within two months; combined with severe respiratory, circulation, hepatic or renal dysfunction, upper gastrointestinal bleeding caused by liver cirrhosis, gastric cancer, acute gastric mucosal lesions, etc.; pregnant or breastfeeding women; bad addictions (such as alcohol and drug abuse); mental illness or with impaired consciousness.
Relevant medical information of patients in Group A and Group B were collected including gender, age, whether they have taken NSAIDs, helicobacter pylori (Hp) infection, etc.
The urea breath test was used to detect Hp infection. The subjects had to fast for at least 4 hours before detection. After taking off the cap of the bottom air bag, the subjects blew the air bag full and tightened the cap. Then they took a urea (14C) capsule. After resting for 15 minutes, they blew again with a gas collecting card in the mouth. They could breathe during the blowing process, and they stopped when the gas collecting card changed from orange to yellow. The subjects’ gas collection card was detected by 14C detector, and Hp was positive when the judgment value was equal to or greater than 50.
Upon admission, 3 mL venous blood was drawn from the subjects, and centrifuged to obtain serum. Enzyme-linked immunosorbent assay (ELISA) was used to detect serum EGF levels, and radioimmunoassay was used to determine serum GAS levels.
Data was analysed using SPSS 25.0. Kolmogorov-Smirnov test was used to test the normality of all measurement data. Measurement data such as age, serum GAS, serum EGF were expressed as mean ±SD, and were compared by independent sample t-test between the two groups. Categorical data were expressed as frequencies and percentages. Categorical data between the two groups were compared by Chi-square test. Logistic regression analysis was used to analyse the risk factors of gastric ulcer complicated with acute upper gastrointestinal bleeding. The value of serum GAS, EGF in early diagnosis of gastric ulcer with upper gastrointestinal bleeding was evaluated by receiver operating characteristic (ROC) curve. A p-value less than 0.05 was regarded as statistically significant.
RESULTS
The results of univariate analysis showed that there were statistically significant differences between Group A and Group B in taking NSAIDs, Hp infection, serum GAS and EGF (all p <0.001,Table I); however, there was no significant difference in gender, age, smoking and drinking groups (p = 0.692, 0.660, 0.163 and 0.097, respectively, Table I).
Table I: Comparison of related parameters in the two groups.
|
Index |
Group A (n=125) |
Group B (n=125) |
p-value |
|
Gender [male, n (%)] |
79 (63.20) |
82 (65.60) |
0.692 |
|
Age (years) |
58.08±9.48 |
57.56±9.20 |
0.660 |
|
Smoking [n (%)] |
73 (58.40) |
62 (49.60) |
0.163 |
|
Drinking [n (%)] |
77 (61.60) |
64(51.20) |
0.097 |
|
Taking NSAIDs [n (%)] |
106 (84.80) |
70 (56.00) |
<0.001 |
|
Hp infection [n (%)] |
114 (91.20) |
88 (70.40) |
<0.001 |
|
Serum GAS (μg/L) |
94.33±15.32 |
123.67±19.78 |
<0.001 |
|
Serum EGF (pg /mL) |
100.75±16.41 |
138.50±22.15 |
<0.001 |
Logistic regression analysis was performed on factors such as taking NSAIDs (yes=1, no=0), Hp infection (yes=1, no=0), serum GAS (actual test value), serum EGF (actual test value), and the results showed that serum GAS and serum EGF were independent risk factors for gastric ulcer and upper gastrointestinal bleeding (both p <0.001, Table II).
Table II: Multivariate logistic regression analysis of gastric ulcer and upper gastrointestinal bleeding.
|
Risk factor |
B |
S.E. |
p-value |
Exp (B) |
|
Taking NSAIDs |
-0.968 |
1.013 |
0.339 |
0.380 |
|
Hp infection |
-0.908 |
0.980 |
0.354 |
0.403 |
|
Serum GAS |
2.124 |
0.328 |
<0.001 |
8.361 |
|
Serum EGF |
-1.864 |
0.283 |
<0.001 |
0.155 |
The ROC area of serum GAS to predict gastric ulcer and acute upper gastrointestinal bleeding was 0.774 (95% CI: 0.714 - 0.835, p<0.001). When serum GAS was ≤98.09 μg/L, its sensitivity to predict gastric ulcer and acute upper gastrointestinal bleeding was 84.8%, and the specificity was 56%.
The ROC area of serum EGF to predict gastric ulcer and acute upper gastrointestinal bleeding was 0.810 (95% CI: 0.753 - 0.867, p<0.001). When serum EGF was ≤109.95 pg/mL, its sensitivity to predict gastric ulcer and acute upper gastrointestinal bleeding was 84.8%, and the specificity was 68.8%, as shown in Figure 1.
The ROC area of serum EGF to predict gastric ulcer and acute upper gastrointestinal bleeding was 0.810 (95% CI: 0.753 - 0.867, p <0.001), higher than ROC area of serum GAS.
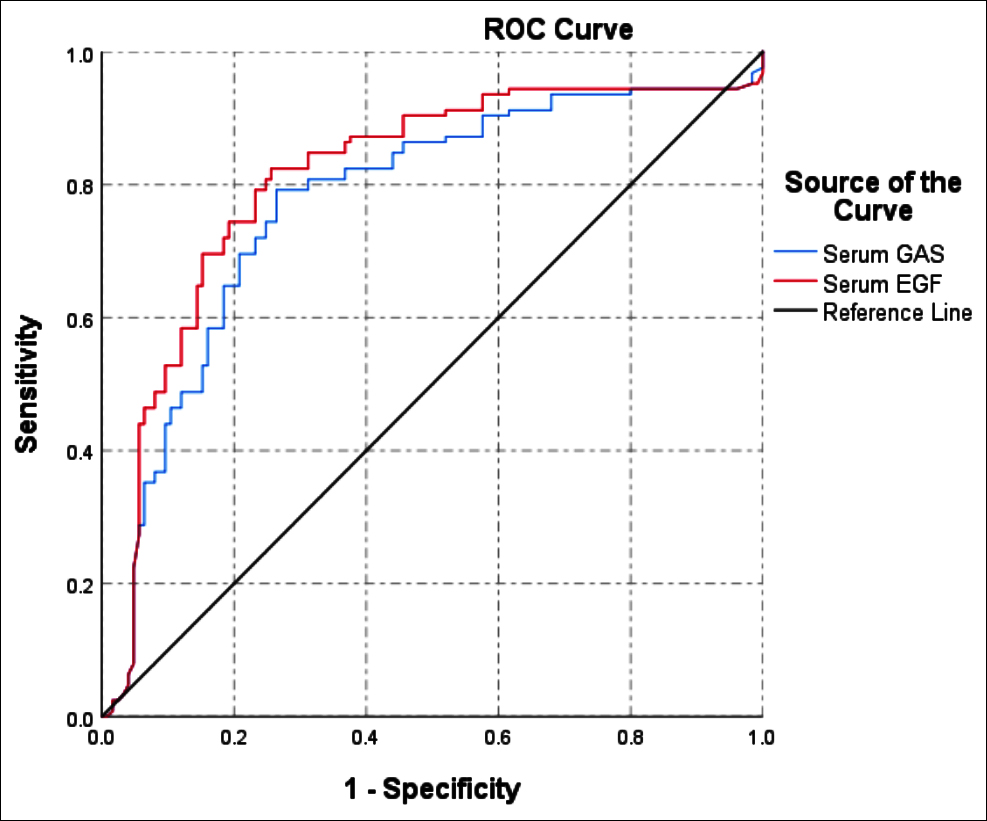 Figure 1: ROC curves of serum GAS and EGF to predict gastric ulcer and acute upper gastrointestinal bleeding.
Figure 1: ROC curves of serum GAS and EGF to predict gastric ulcer and acute upper gastrointestinal bleeding.
DISCUSSION
Hp infection and taking NSAIDs are two important pathogenic factors of peptic ulcer.10,11 Numerous studies have confirmed that Hp and NSAIDs are two independent gastric mucosal damaging factors, and can cause gastric mucosal damage through different mechanisms.12,13
NSAIDs mainly inhibit the synthesis of prostaglandins in gastric mucosa, thus inhibiting the secretion of mucus and bicarbonate in gastric epithelial cells, reducing the blood flow of gastric mucosa and the renewal rate of gastric epithelium, stimulating the adhesion of neutrophils in blood vessel wall, and migrating out of blood vessels to infiltrate locally, causing gastric mucosa damage.14 However, the role of Hp infection in ulcer with hemorrhage has not yet been completely defined. A study has found that Hp infection increases the risk of bleeding peptic ulcer disease.15
The results showed that there was a statistically significant difference between Group A and Group B in taking NSAIDs and Hp infection in univariate analysis. Multivariate logistic regression analysis showed that Hp infection and recent NSAIDs taken history were not independent risk factors for gastric ulcer combined with upper gastrointestinal bleeding. However, Chen et al. found that Hp infections were independent ulcer risk factors using multiple logistic regression analysis.16 Previous studies have concluded that among patients with peptic ulcer and upper gastrointestinal bleeding, the Hp positive infection rate is significantly higher than that of patients without upper gastrointestinal bleeding.17
According to a study, serum GAS value was closely related to the disease process of peptic ulcer and was of prognostic value.18 Research showed that,except for Zollinger-Ellison syndrome, a fasting serum GAS level could not be used as a screening test for peptic ulcer disease in children.19 The results of this study showed that the difference in serum GAS between Group A and Group B was statistically significant. Multivariate logistic regression analysis showed that serum GAS was an independent risk factor for gastric ulcer and acute upper gastrointestinal bleeding. ROC curve analysis showed that the area under the curve of serum GAS to predict gastric ulcer and acute upper gastrointestinal bleeding was 0.774 (95% CI: 0.714-0.835, p<0.001). When serum GAS was ≤98.09 μg/L, its sensitivity to predict gastric ulcer and acute upper gastrointestinal bleeding was 84.8%, and the specificity was 56%.
One study showed that EGF was important for gastric ulcer healing.20 Serum EGF levels may be of closely relation between gut hormones and peptic ulcer.21 The results of this study showed that there was a statistically significant difference in serum EGF between Group A and Group B. Multivariate logistic regression analysis showed that serum EGF was an independent risk factor for gastric ulcer and acute upper gastrointestinal bleeding.
ROC curve analysis showed that the area under the curve of serum EGF to predict gastric ulcer and acute upper gastrointestinal bleeding was 0.810 (95% CI: 0.753-0.867, p<0.001). When serum EGF was ≤109.95 pg/mL, its sensitivity to predict gastric ulcer and acute upper gastrointestinal bleeding was 84.8%, and the specificity was 68.8%. The area under the curve of serum EGF to predict gastric ulcer complicated with upper gastrointestinal hemorrhage was larger than that of serum GAS, and its predictive value was greater.
CONCLUSION
Serum GAS and EGF are independent risk factors for gastric ulcer complicated with acute upper gastrointestinal bleeding. The predictive value of serum GAS and EGF is high for gastric ulcer complicated with acute upper gastrointestinal bleeding, and the predictive value of serum EGF is greater than that of serum GAS.
ETHICAL APPROVAL:
This study was conducted with the approval from the Ethics Committee of the Beijing Jiangong Hospital, China.
PATIENTS’ CONSENT:
All patients signed a document of informed consent.
CONFLICT OF INTEREST:
The authors have no conflicts of interests to declare.
AUTHORS’ CONTRIBUTION:
YL: Interpretation of data for the work, drafting the work.
YS: Revising it critically for important intellectual content, final approval of the version to be published.
REFERENCES
- Matsueda K, Kawano S, Okada H. Primary localised amyloidosis of the stomach mimicking healing gastric ulcer. Gastrointes Endosc 2020; 91(4):947-8. doi: 10.1016/j.gie. 2019.12.004.
- Ardalani H, Hadipanah A, Amirhossein S. Medicinal plants in the treatment of peptic ulcer disease: A review. Mini Rev Med Chemis 2020; 20(8):662-702. doi: 10.2174/1389 557520666191227151939.
- Hreinsson JP, Kalaitzakis E, Gudmundsson S, Björnsson ES. Upper gastrointestinal bleeding: incidence, etiology and outcomes in a population-based setting. Scand J Gastroenterol 2013; 48(4):439-7. doi: 10.3109/00365521. 2012. 763174.
- Laursen SB, Gralnek IM, Stanley AJ. Raising the threshold for hospital admission and endoscopy in upper gastrointestinal bleeding during the COVID-19 pandemic. Endoscopy 2020; 52(10):930-1. doi: 10.1055/a-1202- 1374.
- Chiang CH, Jeng JE, Wang WM, Jheng BH. A comparative study of three fecal occult blood tests in upper gastrointestinal bleeding. Kaohsiung J Med Sci 2006; 22(5):223-8. doi: 10.1016/S1607-551X(09)70240-2.
- Han H, Sun X, Zheng X, Xiao J. Effects of somatostatin combined with erythromycin on gastrointestinal function recovery, gastrointestinal hormone levels and immune indexes in patients after gastrointestinal surgery. Acta Microscopica 2020; 29 (3):1454-60.
- Kang J, Kang LL, Yu H, Tao C, Chen LM. Levels and significance of serum peptide hormone and monoamine neurotransmitter in patients with gastric ulcer. Progress Modern Biomedicine 2016; 16(26):5131-4.
- Chen W, Wu D, Jin Y, Li Q, Liu Y, Qiao X, et al. Pre-protective effect of polysaccharides purified from Hericium erinaceus against ethanol-induced gastric mucosal injury in rats. Int J Biol Macromol 2020; 159:948-56. doi: 10.1016/j.ijbiomac. 2020.05.163.
- Liu X, Li J, Li Y, Zhang H, Zhang H. The micro mechanism and nursing of the treatment of gastric ulcer combined with gastric hemorrhage with aluminum magnesium carbonate. Acta Microscopica 2020; 29(3):1598-606.
- Zahid R, Akram M, Riaz M, Munir N, Shehzad M. Phytotherapeutic modalities for the management of Helicobacter pylori associated peptic ulcer. Eur J Inflamm 2020; 18:1-16. doi.org/10.1177/2058739220968308.
- Jones MP. The role of psychosocial factors in peptic ulcer disease: Beyond helicobacter pylori and NSAIDs. J Psychoso Res 2006; 60(4):407-12. doi: 10.1016/j.jpsychores.2005. 08.009.
- Abdulateef MM, Abdulameer RA, Muhsun LH. Prevalence of helicobacter pylori infection in patients with upper gastrointestinal bleeding. Eur J Preven Med 2019; 7(6): 123-9.
- Laporte J R, Ibanez L, Vidal X, Vendrell L, Leone R. Upper gastrointestinal bleeding associated with the use of NSAIDs. Drug-Safety 2004; 27(6):411-20. doi: 10.2165/ 00002018-200427060-00005.
- Sugisaki N, Iwakiri R, Tsuruoka N, Sakata Y, Shimoda R, Fujimoto S, et al. A case-control study of the risk of upper gastrointestinal mucosal injuries in patients prescribed concurrent NSAIDs and antithrombotic drugs based on data from the Japanese national claims database of 13 million accumulated patients. J Gastroenterol 2018; 53(12): 1253-60. doi: 10.1007/s00535-018-1483-x.
- Hung KW, Knotts RM, Faye AS, Pont AR, Lebwohl B, Abrams JA, et al. Factors associated with adherence to helicobacter pylori testing during hospitalisation for bleeding peptic ulcer disease. Clin Gastroenterol H 2020; 18(5):1091-8. doi: 10.1016/j.cgh.2019.07.037.
- Chen M, Wu M, Lee W, Wang H. A multiple logistic regression analysis of risk factors in different subtypes of gastric ulcer. Hepato-gastroenterol 2002; 49(44):589-92.
- Vestergård A, Bredahl K, de Muckadell OB, Pedersen OB, Hansen JM. Bleeding peptic ulcer. Prevalence of Helicobacter pylori and use of nonsteroidal anti-inflammatory drugs/ acetylsalicylic acid. Ugeskrift for Laeger 2009; 171(4):235-9.
- Wang JP. Clinical significance of changes of serum GAS, TNF-α and TGF-α levels after treatment in patients with peptic ulcer. J Huaihai Med 2009; 27(6):502-3.
- Tsai YH, Chang MH. Serum gastrin levels in children with peptic ulcer disease. J Formos Med Assoc 1992; 91(3): 283-6.
- Fagundes FL,Piffer GM,Périco LL,Rodrigues VP,Hiruma-Lima CA,dos Santos RC. Chrysin modulates genes related to inflammation, tissue remodeling, and cell proliferation in the gastric ulcer healing. Int J Mol Sci 2020; 21(3):760-72. doi: 10.3390/ijms21030760.
- Tang X, Han P, Chen Z. Changes of serum GAS, MTL, EGF AND CGRP level in patients with duodenal ulcer before and after treatment. J Guangxi Med Univ 1998; 15(2):8